Can Dietary Prebiotics Reduce the Rewarding Effects of High-Calorie Foods?

- An experimental study of overweight young adults published in Gut demonstrated that dietary prebiotics can reduce the rewarding effects of high-calorie food
- After 14 days of using prebiotics, fMRI scans showed reduced reward-related brain activation in response to high-calorie food compared to the placebo group
- Occurrence of short-chain-fatty-acid-producing Bifidobacteriaceae increased in participants taking prebiotics
We are all familiar with the intense feelings of pleasure we get after consuming highly tasty and palatable food – a piece of chocolate cake, for example. We will remember these feelings and associate them with this food. When we decide what to eat in the future, we will recall the memories of that food and the feelings we experienced when eating it —and likely develop a desire to eat the same food again. That is an important part of how we create our food preferences.
Consuming highly palatable foods, especially those high in sugar and fat, can increase dopamine in the brain, creating a sense of pleasure and satisfaction
Rewarding effects of food
The feelings of pleasure we experience when eating highly palatable foods are created by our brain’s reward system. The brain’s reward system comprises a complex network of neural circuits that play a fundamental role in motivation, reinforcement, and pleasure. This process involves the release of neurotransmitters, particularly dopamine, in response to rewarding stimuli, such as food, sex, or positive social interactions. This dopamine release reinforces behaviors associated with the reward, creating a drive to seek out and repeat those behaviors (Lewis et al., 2021) (see Figure 1).
Consuming highly palatable foods, especially those high in sugar and fat, can increase dopamine in the brain, creating a sense of pleasure and satisfaction. This activation of reward neural pathways reinforces the desire to eat, making us more likely to seek those foods in the future.
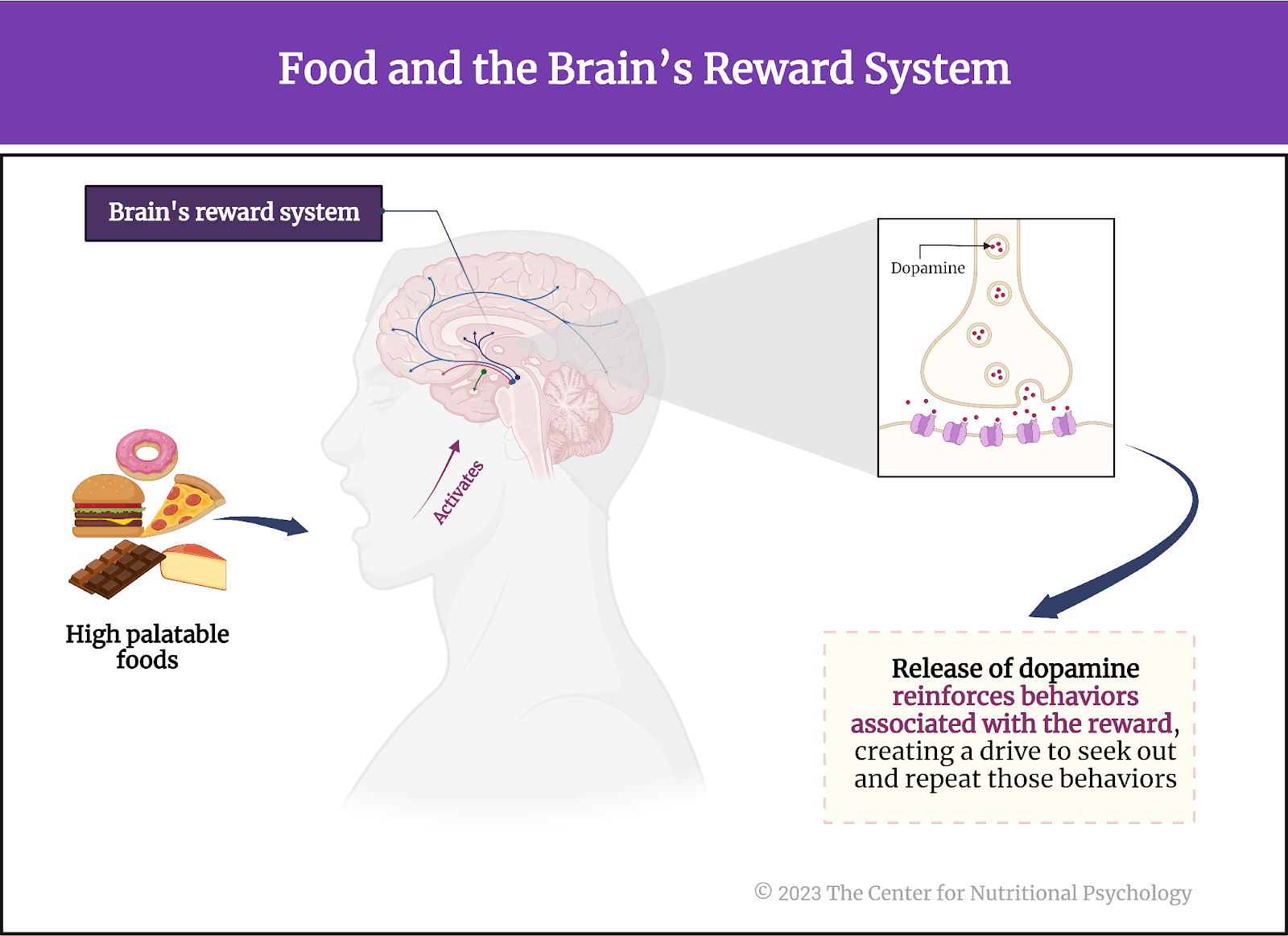
Figure 1. Food and the brain’s reward system
How is the brain’s reward system linked to obesity?
Repeated exposure to highly rewarding foods, especially in excess, can lead to desensitization of the reward system. When this happens, an individual will need more and more food to achieve the expected pleasant feelings, i.e., the rewarding effects of food. This might make the individual increase the intake of the food in question, potentially leading to overeating and an increase in body weight in the long run (see Figure 2).
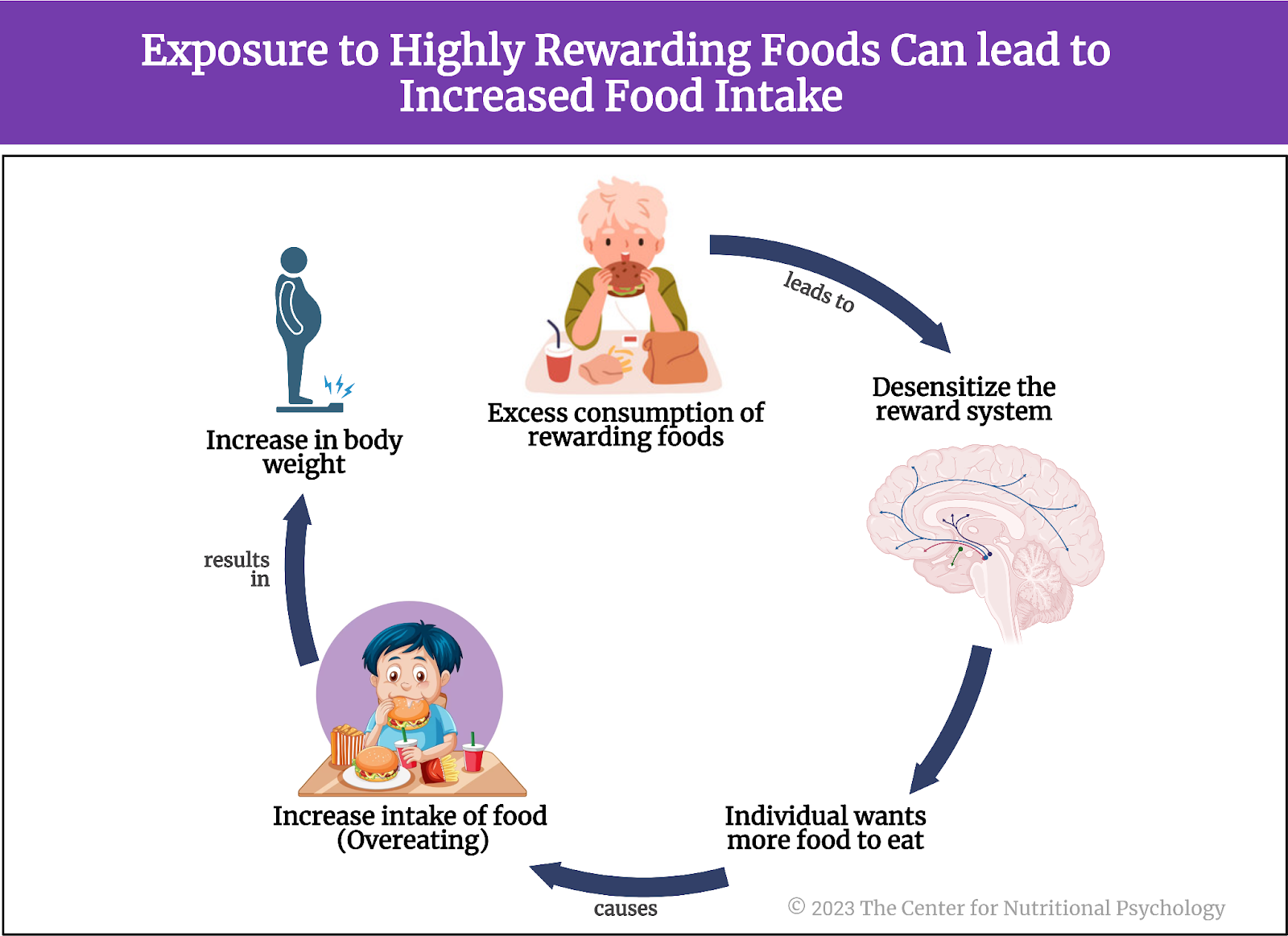
Figure 2. Exposure to highly rewarding foods can lead to increased food intake
The described mechanism is similar to that which occurs with addictions. This is the reason why some authors propose that an individual can develop an addiction to certain foods and that food addiction should be recognized as a distinct mental health condition. Studies these researchers carried out indicate that consumption of foods rich in both sugar and fats, such as (many types of) ultra-processed foods, is particularly likely to result in food addiction (Gearhardt et al., 2011, 2023; Hedrih, 2023; Thanarajah et al., 2023).
Studies of these neural mechanisms in humans found functional alterations in the areas of the brain involved in the reward system in overweight individuals (e.g., Pujol et al., 2021; Seabrook et al., 2023), while studies on mice clearly show that high-fat diets can indeed induce obesity by disrupting the functioning of neural mechanisms regulating food intake (e.g., Ikemoto et al., 1996).
This leads many researchers to search for methods of influencing the brain’s reward system to prevent obesity or help individuals regain healthy body mass.
The current study
Study author Evelyn Medawar and her colleagues hypothesized that consuming high doses of prebiotic fiber as a supplement would change the patterns of neural activity related to food in the brain reward system in individuals at risk for weight gain and developing diabetes. Prebiotic fiber is a type of dietary fiber that promotes the growth and activity of beneficial bacteria in the gut, supporting a healthy microbiota. While it is indigestible for humans, it serves as food for various bacteria living in the gut, promoting their growth.
The authors of this study expected that an intervention using prebiotics would alter the composition of the gut microbiome, which would, in turn, alter the food-related patterns of brain activation (Medawar et al., 2023).
The gut microbiome or gut microbiota is the community of trillions of bacteria, fungi, viruses, and other microorganisms that live in the human gut. These microorganisms are critical for digestion, allowing the human digestive system to digest substances it would not be able to digest without them. Still, they also serve a number of other metabolic roles.
High doses of prebiotic fiber as a supplement would change the patterns of neural activity related to food in the brain reward system
How can gut bacteria affect the brain reward system?
Scientists studying human life processes in recent decades discovered a system in the human body that allows communication between gut microorganisms and the brain. This bidirectional communication system is called the microbiota-gut-brain axis (MGBA). It links the gut microbiota, the gastrointestinal tract, and the central nervous system (CNS).
Aside from their role in digestion, gut microorganisms produce various signaling molecules that can influence neural, endocrine, and immune functions. These molecules enable communication between the gut and the brain through neural, hormonal, and immunological pathways. Studies have already identified various ways in which processes in the gut can affect brain functioning, but much still remains to be discovered (Qu et al., 2023; Valles-Colomer et al., 2019; Zhu et al., 2023).
Recent findings indicate that the microbiota-gut-brain axis MGBA can affect and regulate the reward system of the brain as well (Carbia et al., 2023; García-Cabrerizo et al., 2021). Given this, it is possible that gut microbiota changes could also affect the brain’s reward system (see Figure 3).
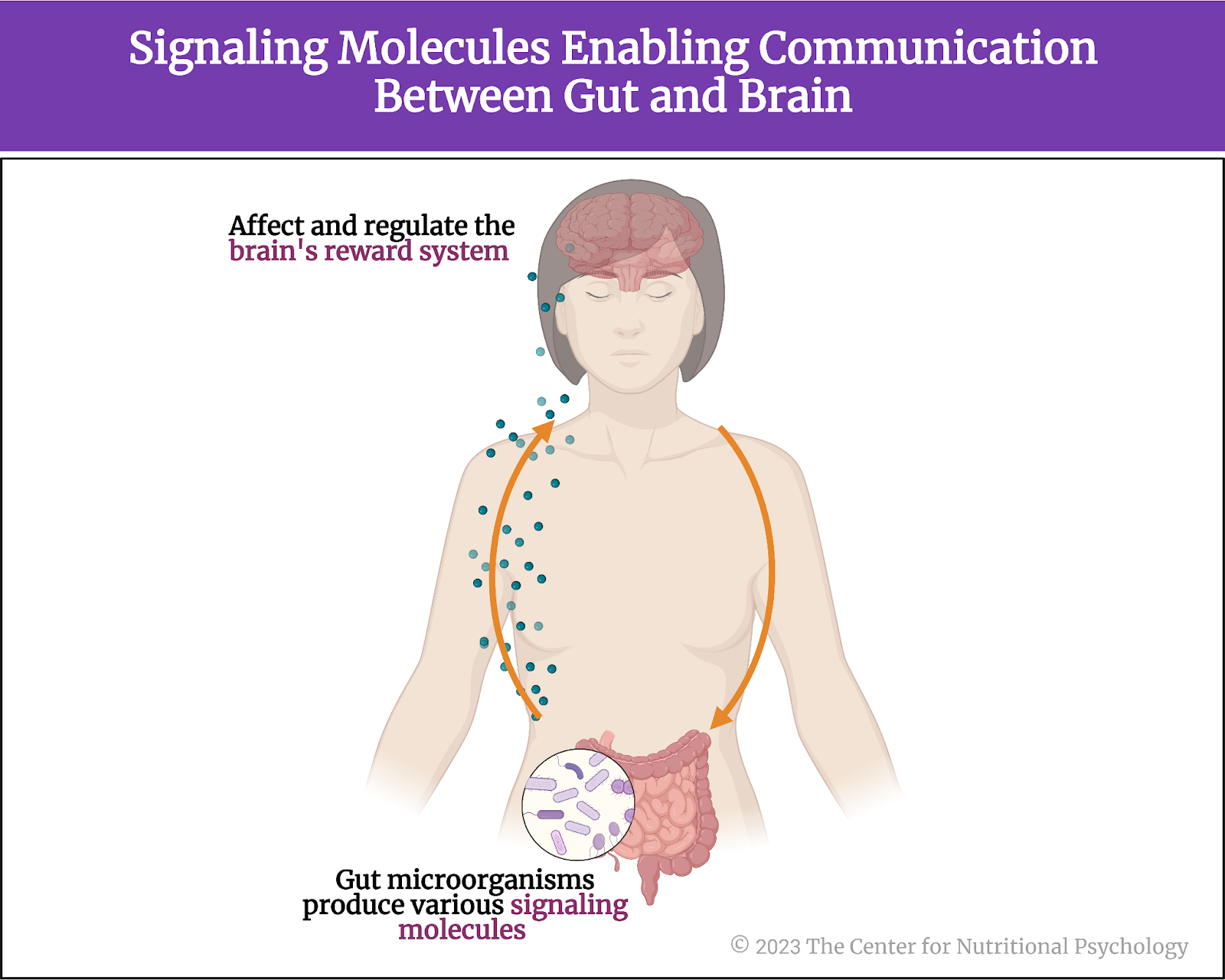
Figure 3. Signaling molecules enabling communication between the gut and brain
Recent findings indicate that the microbiota-gut-brain axis (MGBA) can affect and regulate the reward system of the brain
The procedure
Study participants were 59 overweight adults (body mass index values between 25 and 30). Their ages ranged between 18 and 42 years. Nineteen of them were females. Participants received 9-10 EUR per hour for their activities during testing days and another 30 EUR for completing the study.
The study’s authors prepared two different treatment substances for participants: a prebiotic fiber supplement and a placebo. The daily dose of prebiotic fiber consisted of 30 grams of inulin, a carbohydrate found in plants such as chicory root. Humans lack the enzymes needed to digest inulin, but many beneficial bacteria in the gut can ferment it. The product of this fermentation is short-chain fatty acids. Among other things, these substances are involved in the functioning of the MGBA (learn more about short-chain fatty acids in NP 120 Parts I and II through CNP) (see Figure 4).
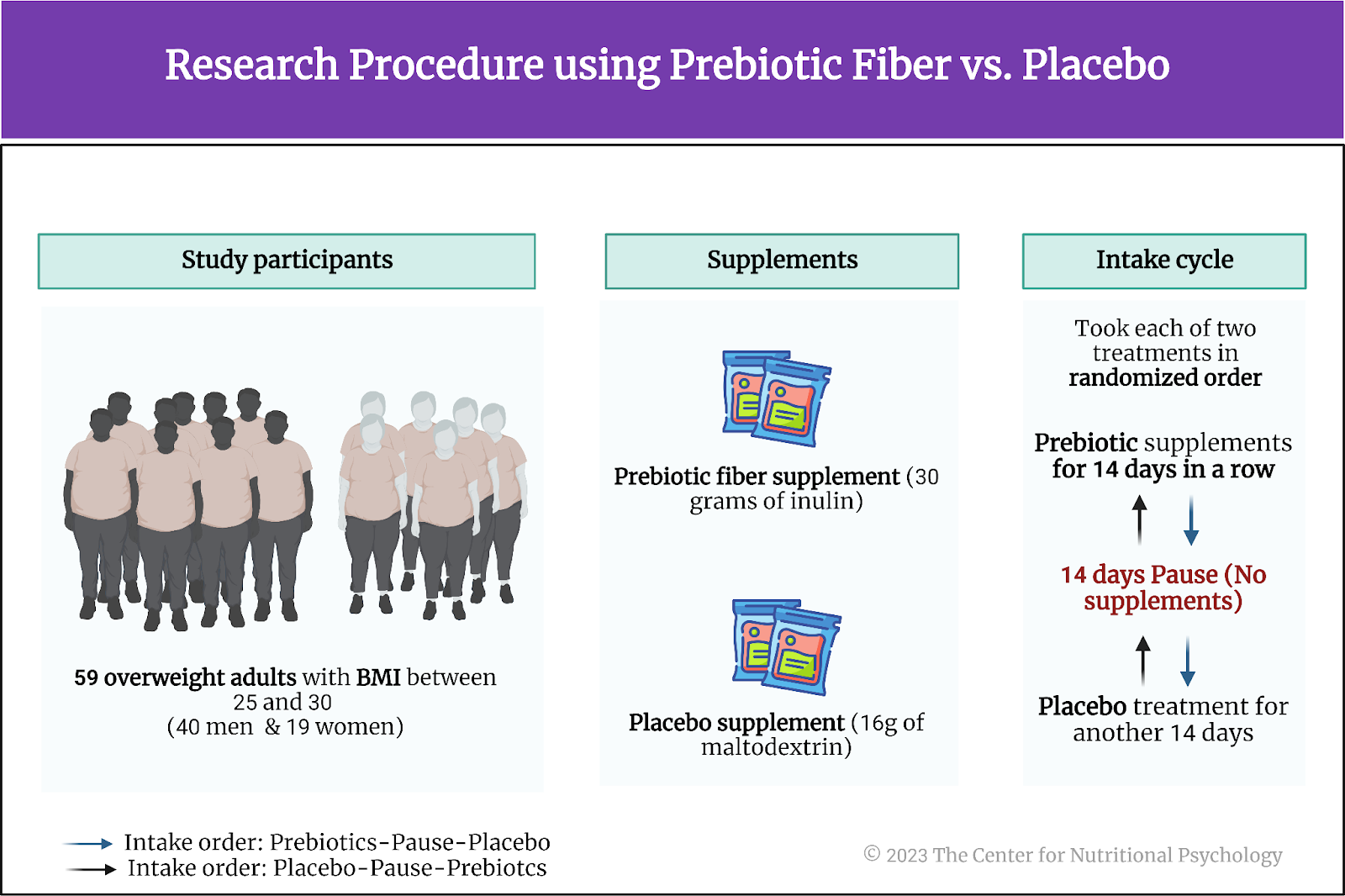
Figure 4. Research procedure using prebiotic fiber vs. placebo
A daily placebo treatment substance consisted of 16g of maltodextrin, a carbohydrate used as a food additive and a thickening agent derived from starch. The placebo treatment was matched on calories with the prebiotic fiber treatment. Both substances were packed in identical sachets, so participants could not tell which substance a sachet contained. In both cases, participants were supposed to take two sachets of the treatment substance daily.
Each participant was supposed to take each of the two treatment substances for 14 days in a row, with two weeks between the treatments. The order in which each participant would take the treatments was randomized – some participants first took the prebiotic fiber for 14 days, paused for 14 days, and then took a placebo for another 14 days. Other participants would start with 14 days of placebo and finish with 14 days of using the prebiotic fiber. This was a blind study, so study participants and staff working with them did not know when they were taking a placebo and when it was prebiotic fiber.
Neuroimaging and measurements
Before and after each 14-day treatment, participants underwent functional magnetic resonance imaging (fMRI). During imaging, they viewed pictures of food and art and rated how much they wanted the item shown in the picture. Researchers told them they would receive a reward based on their ratings – either a food item that could be eaten immediately after fMRI or a carton print of an art piece.
Participants gave blood samples to assess short-chain fatty acid concentrations, gastrointestinal hormones, glucose, lipids, and inflammatory markers. They also gave stool samples for measuring gut microbiota composition and short-chain fatty acids.
Brain reactions to high-calorie foods decreased after prebiotic treatment
On average, during fMRI scans, participants wanted food more than they wanted art. The prebiotic treatment did not change the relationship between these wanting scores. However, after 14 days of taking prebiotics, participants had lower overall wanting scores than after 14 days of taking a placebo. In particular, after prebiotics treatment, participants expressed very little desire for very low and very high-calorie foods and plants (see Figure 5).
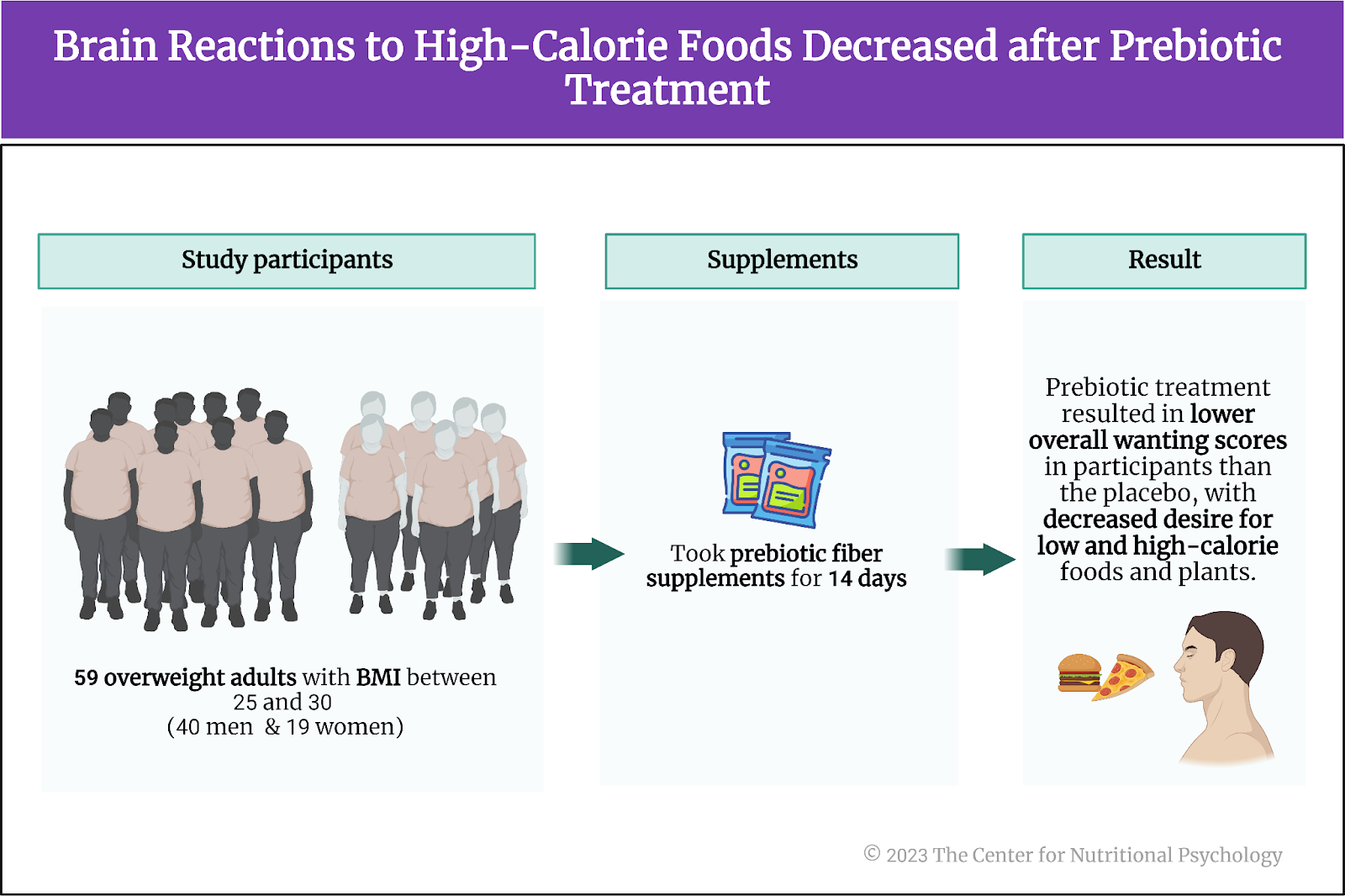
Figure 5. Brain reactions to high-calorie foods decreased after prebiotic treatment
When brain activity was examined, regional brain activity in reaction to food and art pictures remained the same. Evaluation of food pictures activated large parts of the reward network, including the ventral tegmental area, hypothalamus, nucleus accumbens, basal ganglia, and ventromedial thalamus, as well as anterior insula, amygdala, cingulate, ventromedial prefrontal cortex, and distinct parts of the orbitofrontal cortex.
However, brain reactions to pictures of wanted, high-calorie food decreased after prebiotics (compared to placebo) in three brain regions – ventral tegmental area, right and right medial the orbitofrontal cortex. Additionally, participants reported feeling less subjective hunger during fMRI after the prebiotics treatment.
Brain reactions to pictures of wanted, high-calorie food decreased after prebiotics compared to placebo
Prebiotic treatment altered gut microbiota composition
Analysis of stool samples indicated that the treatment with prebiotics led to significant shifts in gut microbiota composition. Overall richness, evenness, and diversity (the number of different species of microorganisms) decreased. The amounts of Bifidobacteria in samples increased. Increases in Bifidobacteria were associated with increases in the activity of a number of metabolic pathways.
Participants who had a more substantial decrease in Subdoligranulum bacteria after prebiotics treatment tended to show a decreased activation of the brain’s ventral tegmental area in response to high-calorie food. Participants who had a decrease in Lactiplantibacillus, a lactic acid-producing bacteria, after prebiotics treatment tended to have decreased activation in the right medial orbitofrontal cortex.
Conclusion
The study showed that intervention with a common prebiotic attenuated reactions of the brain’s reward network in response to high-calorie food. It also induced changes to the gut microbiota, through which this effect was likely achieved.
The study showed that intervention with a common prebiotic attenuated reactions of the brain’s reward network in response to high-calorie food
Since the discovery of the microbiota-gut-brain axis, scientists have considered using prebiotics or other interventions aimed at gut microbiota to achieve beneficial changes in brain activity and behavior. The results of this study indicate that this idea holds merit. While it used a common type of prebiotic, it proved that brain functioning can be affected in this way. This opens a path for future researchers to develop more effective and specific ways to achieve mental health outcomes through interventions aimed at gut microbiota.
The paper “Prebiotic diet changes neural correlates of food decision-making in overweight adults: a randomized controlled within-subject cross-over trial” was authored by Evelyn Medawar, Frauke Beyer, Ronja Thieleking, Sven-Bastiaan Haange, Ulrike Rolle-Kampczyk, Madlen Reinicke, Rima Chakaroun, Martin von Bergen, Michael Stumvoll, Arno Villringer, A Veronica Witte.
References
Carbia, C., Bastiaanssen, T. F. S., Iannone, F., García-cabrerizo, R., Boscaini, S., Berding, K., Strain, C. R., Clarke, G., Stanton, C., Dinan, T. G., & Cryan, J. F. (2023). The Microbiome-Gut-Brain axis regulates social cognition & craving in young binge drinkers. EBioMedicine, (In press), 104442. https://doi.org/10.1016/j.ebiom.2023.104442
García-Cabrerizo, R., Carbia, C., O´Riordan, K. J., Schellekens, H., & Cryan, J. F. (2021). Microbiota-gut-brain axis as a regulator of reward processes. Journal of Neurochemistry, 157(5), 1495–1524. https://doi.org/10.1111/JNC.15284
Gearhardt, A. N., Bueno, N. B., DiFeliceantonio, A. G., Roberto, C. A., Jiménez-Murcia, S., & Fernandez-Aranda, F. (2023). Social, clinical, and policy implications of ultra-processed food addiction. BMJ, e075354. https://doi.org/10.1136/bmj-2023-075354
Gearhardt, A. N., Yokum, S., Orr, P. T., Stice, E., Corbin, W. R., & Brownell, K. D. (2011). Neural Correlates of Food Addiction. Archives of General Psychiatry, 68(8), 808–816. https://doi.org/10.1001/ARCHGENPSYCHIATRY.2011.32
Hedrih, V. (2023). Scientists Propose that Ultra-Processed Foods be Classified as Addictive Substances. CNP Articles in Nutritional Psychology. https://www.nutritional-psychology.org/scientists-propose-that-ultra-processed-foods-be-classified-as-addictive-substances/
Ikemoto, S., Takahashi, M., Tsunoda, N., Maruyama, K., Itakura, H., & Ezaki, O. (1996). High-fat diet-induced hyperglycemia and obesity in mice: Differential effects of dietary oils. Metabolism, 45(12), 1539–1546. https://doi.org/10.1016/S0026-0495(96)90185-7
Lewis, R. G., Florio, E., Punzo, D., & Borrelli, E. (2021). The Brain’s Reward System in Health and Disease. In Advances in Experimental Medicine and Biology (Vol. 1344, pp. 57–69). Springer. https://doi.org/10.1007/978-3-030-81147-1_4
Medawar, E., Beyer, F., Thieleking, R., Haange, S.-B., Rolle-Kampczyk, U., Reinicke, M., Chakaroun, R., von Bergen, M., Stumvoll, M., Villringer, A., & Witte, A. V. (2023). Prebiotic diet changes neural correlates of food decision-making in overweight adults: a randomised controlled within-subject cross-over trial. Gut, gutjnl-2023-330365. https://doi.org/10.1136/gutjnl-2023-330365
Pujol, J., Blanco-Hinojo, L., Martínez-Vilavella, G., Deus, J., Pérez-Sola, V., & Sunyer, J. (2021). Dysfunctional Brain Reward System in Child Obesity. Cerebral Cortex, 31, 4376–4385. https://doi.org/10.1093/cercor/bhab092
Qu, Y., Eguchi, A., Wan, X., Ma, L., Chang, L., Shan, J., Yang, Y., Mori, C., & Hashimoto, K. (2023). Repeated use of 3,4-methylenedioxymethamphetamine is associated with the resilience in mice after chronic social defeat stress: A role of gut–microbiota–brain axis. Psychiatry Research, 320(October 2022), 0–9. https://doi.org/10.1016/j.psychres.2022.115020
Seabrook, L. T., Naef, L., Baimel, C., Judge, A. K., Kenney, T., Ellis, M., Tayyab, T., Armstrong, M., Qiao, M., Floresco, S. B., & Borgland, S. L. (2023). Disinhibition of the orbitofrontal cortex biases decision-making in obesity. Nature Neuroscience, 26(1), 92–106. https://doi.org/10.1038/s41593-022-01210-6
Thanarajah, S. E., Difeliceantonio, A. G., Albus, K., Br, J. C., Tittgemeyer, M., Small, D. M., Thanarajah, S. E., Difeliceantonio, A. G., Albus, K., Kuzmanovic, B., & Rigoux, L. (2023). Habitual daily intake of a sweet and fatty snack modulates reward processing in humans. Cell Metabolism, 35, 1–14. https://doi.org/10.1016/j.cmet.2023.02.015
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., Schiweck, C., Kurilshikov, A., Joossens, M., Wijmenga, C., Claes, S., Van Oudenhove, L., Zhernakova, A., Vieira-Silva, S., & Raes, J. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology, 4(4), 623–632. https://doi.org/10.1038/s41564-018-0337-x
Zhu, X., Sakamoto, S., Ishii, C., Smith, M. D., Ito, K., Obayashi, M., Unger, L., Hasegawa, Y., Kurokawa, S., Kishimoto, T., Li, H., Hatano, S., Wang, T. H., Yoshikai, Y., Kano, S. ichi, Fukuda, S., Sanada, K., Calabresi, P. A., & Kamiya, A. (2023). Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nature Immunology. https://doi.org/10.1038/s41590-023-01447-8








Leave a comment