Does Sleep Deprivation Increase Desire for High-Calorie foods?

Listen to this Article
- A study published in Nature Communications found that sleep deprivation decreases the activity of frontal and insular cortices, parts of the human brain responsible for higher-order processes when making food choices.
- At the same time, the activity of the amygdala region, responsible for processing emotions, increases.
- When we lack sleep, food choices become less rational and more emotional, increasing the desire for high-calorie foods and potentially leading to weight gain.
Our minds don’t function as well when we continually get less sleep than we need. After a prolonged period of time with less sleep, concentrating becomes progressively more difficult, our reactions become slower, and our minds can often wander off. We may even fall asleep unintentionally. Our mind starts “drifting.“ Sometimes, consuming substances like coffee, similar caffeinated beverages, or even medications like modafinil (Wingelaar-Jagt et al., 2023) can help us remain vigilant for some additional time. However, we must eventually have a sufficiently long sleep period to recuperate and remain healthy.
Why is sleep important?
Sleep is a natural and recurring state of reduced consciousness and responsiveness, characterized by altered sensory perception and inactivity of voluntary muscles. Sleep consists of two main types: non-REM sleep, which includes four stages characterized by progressively deeper sleep, and REM (rapid eye movement) sleep, a stage associated with vivid dreams and heightened brain activity. During sleep, the body undergoes tissue repair, immune system strengthening, and the consolidation of memories. Sleep is crucial for maintaining physical and mental health. It plays a vital role in regulating mood, cognitive function, and overall well-being.
Insufficient or poor-quality sleep has been linked to various health issues, including impaired immune function, increased risk of chronic conditions, and negative effects on mood and cognitive performance (Hillman & Lack, 2013) (see Figure 1).
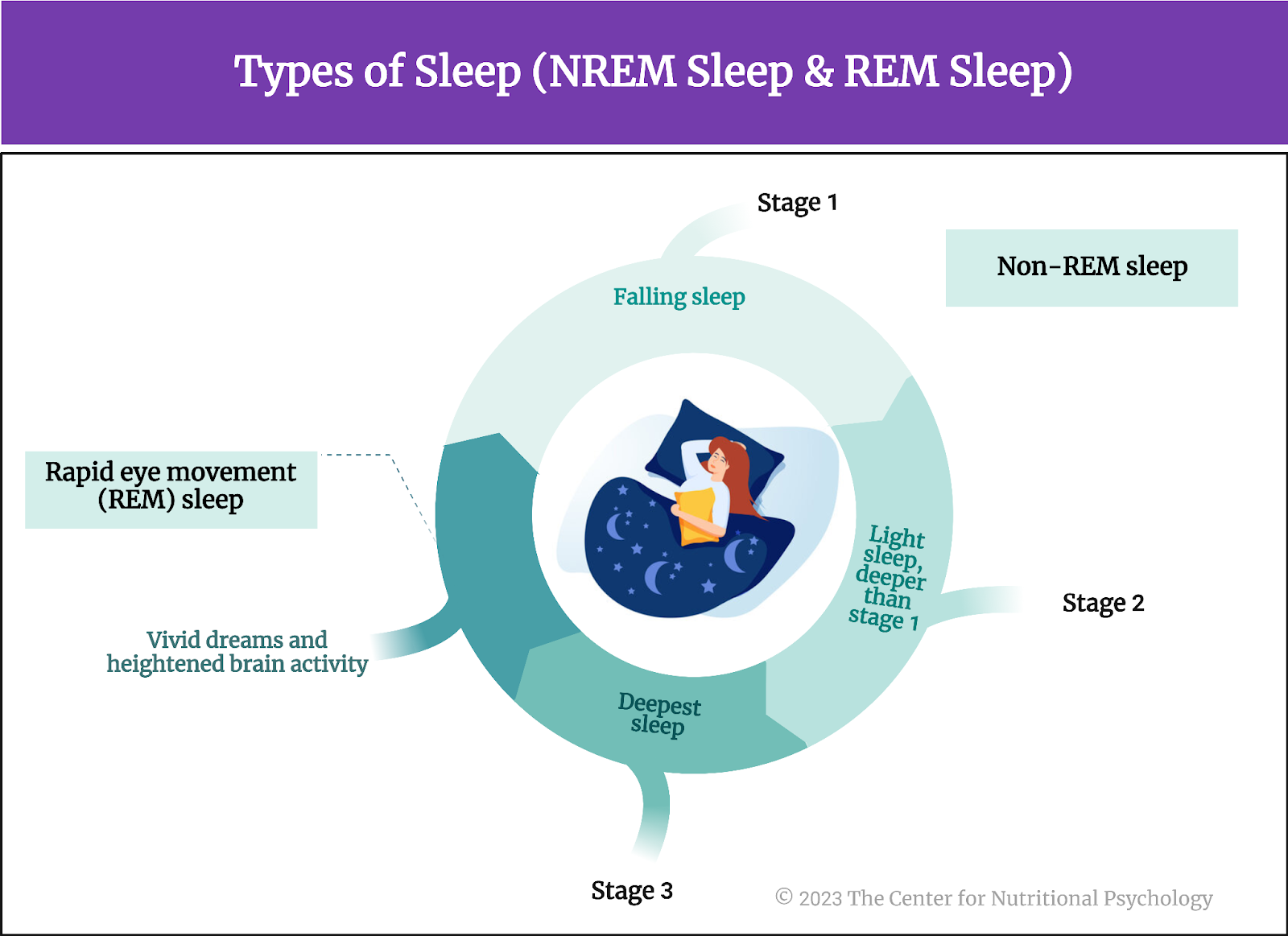
Figure 1. Types of sleep
Lack of sleep is associated with obesity
Studies have reported that sleep loss is one of the risk factors for obesity. This is the case both in children and in adults worldwide. There is mounting evidence that consuming food at night, at the time when we should normally be sleeping, is linked with poor health outcomes, such as worse cardiometabolic health (Bermingham et al., 2023), but also with an increased risk of obesity (Lent et al., 2022). Studies also indicate that food consumed at night tends to be less healthy. Individuals eating at night tend to consume less fruits and vegetables and more sugar-sweetened beverages and fast foods.
There is mounting evidence that consuming food at night, when we should be sleeping, is linked with poor health outcomes
There is an eating disorder called night eating syndrome. Night eating syndrome is a disordered eating pattern characterized by recurrent eating episodes during the night. A person suffering from this disorder typically awakes during the night and starts eating. Individuals with night eating syndrome often consume a significant portion of their daily food during these nocturnal eating episodes. In the morning, they may experience a lack of appetite. Because these eating episodes happen at night, interrupting the normal sleep cycle, the night eating syndrome is also considered a sleep disorder. It affects approximately 1.5% of adults in the U.S., but 9% of patients seeking weight-loss treatments, and 16% of individuals with binge eating disorder (Tzischinsky et al., 2021) (see Figure 2).
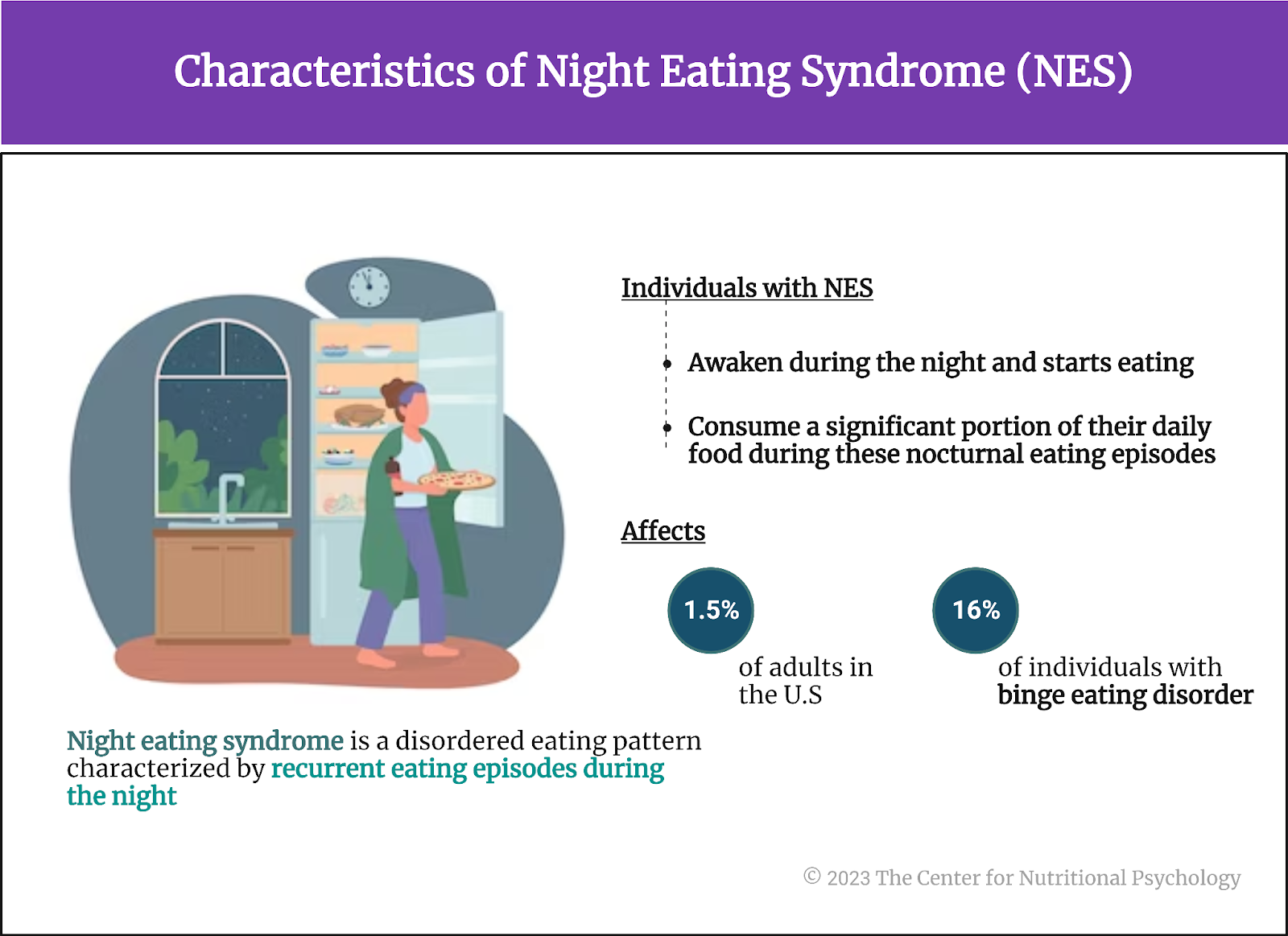
Figure 2. Characteristics of Night Eating Syndrome (NES)
Studies show that in parallel with the rising obesity rates, there is a continued decline in sleep duration in industrialized countries (Greer et al., 2013). What happens to our brain that makes us change our food-related behavior when we lack sleep?
In parallel with the rising obesity rates, there is a continued decline in sleep duration in industrialized countries
The current study
Study author Stephanie M. Greer and her colleagues wanted to find out. There is an abundance of research findings showing that lack of sleep changes our food-related behavior in a way that can lead to weight gain and obesity. Yet, study authors note in spite of this, the neural mechanisms through which this is achieved remain unknown (Greer et al., 2013).
Discovering these mechanisms would allow researchers to understand the link between sleep loss and obesity and potentially devise ways in which individuals could appropriately regulate dietary intake, thus preventing obesity. They conducted a study using functional magnetic resonance imaging (MRI) and focusing on cortical and subcortical regions of the brain, which are instrumental in food desire and evaluating food-related stimuli (see Figure 3).
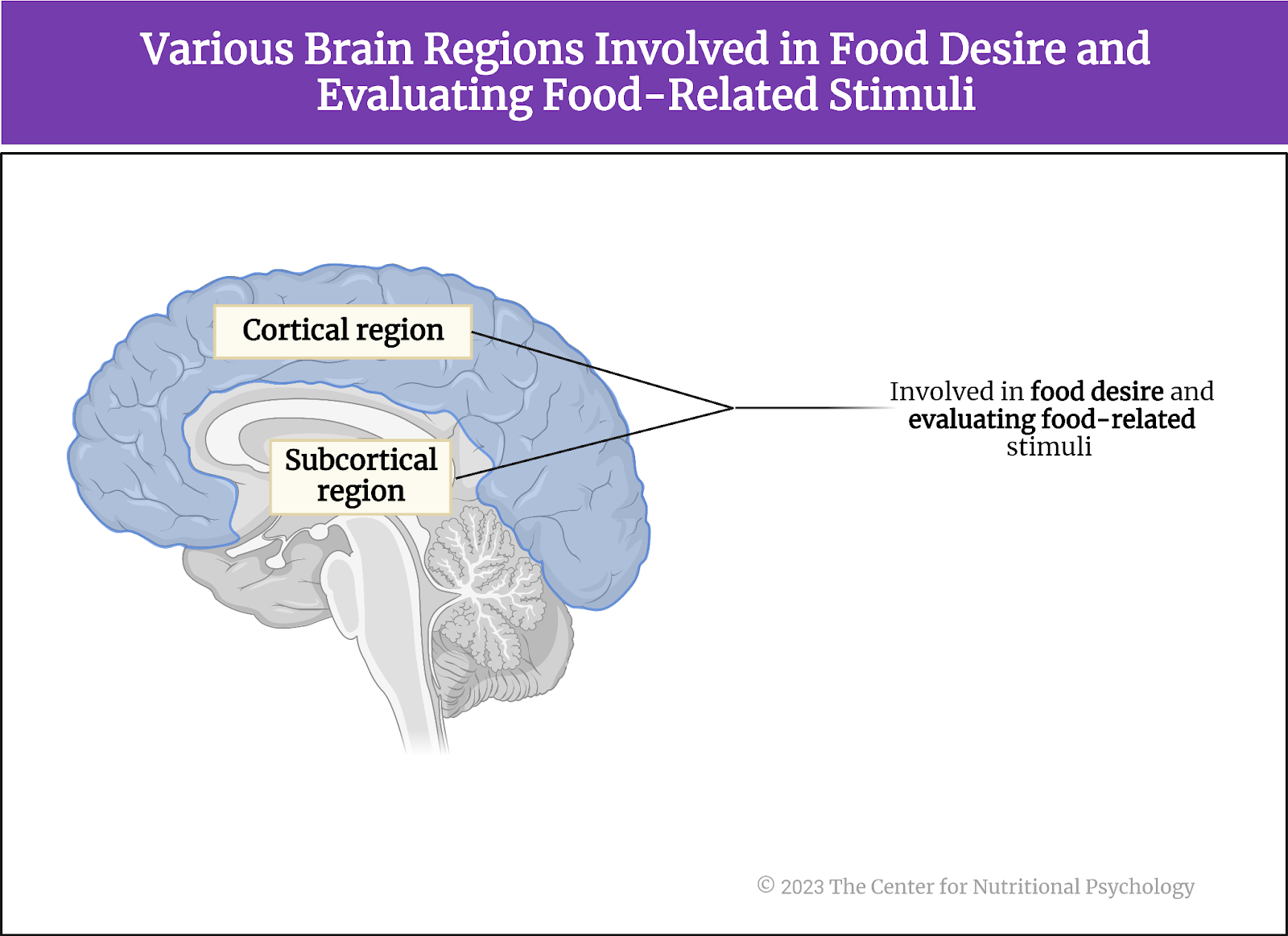
Figure 3. Cortical and subcortical regions of the brain involved in food desire and evaluation
These areas were the anterior insular cortex, lateral orbital frontal cortex, anterior cingulate cortex, amygdala, and the ventral striatum. The three cortex regions all have established roles in determining the value of various food cues, determining our food choices, and integrating various food features (e.g., odor or flavor) to create food preferences. The amygdala and the ventral striatum are strongly implicated in governing our motivation to eat. Activity in the ventral striatum can accurately predict immediate food intake, binge eating, and weight gain.
The study procedure
Study participants were 23 healthy adults who agreed to abstain from drugs, alcohol, and caffeine for three days before the start of the study. They also kept a regular sleep schedule (7-9 hours per night) during this period. Thirteen participants were female. The average age of participants was 21 years, and they were of normal weight.
Each participant completed two experimental sessions – a night of normal sleep in the study authors’ lab monitored by polysomnography equipment and a night of total sleep deprivation (a night during which he/she did not sleep) monitored by lab personnel and by wrist actinography (a device detecting movements of the wrist that can be used to infer whether a person is sleeping). During the sleep deprivation night, participants had a snack around 2:30-3:00 am. The two nights were at least seven days apart.
Functional magnetic resonance imaging and the food-desire task
Participants completed functional magnetic resonance imaging (fMRI) sessions the morning after each experimental night. During the scan, participants completed a food-desire task. The task consisted of 80 pictures of food (without packaging) the researchers collected online. The food items were evenly distributed into five categories – salty, sweet, starchy, fruit, or dairy, and varied in calorie content. The two imaging sessions used the same 80 items with different pictures.
Participants’ task was to rate each food item on a scale ranging from 1 to 4 on how much they wanted that particular food item right now. Researchers did not tell them about the study hypotheses nor the calorie contents of the food items. They told participants that they have two of the shown food items in the lab and that each participant will receive the food item he/she rated higher. Researchers did this to increase the likelihood that participants would rate the food items according to their preferences (see Figure 4).
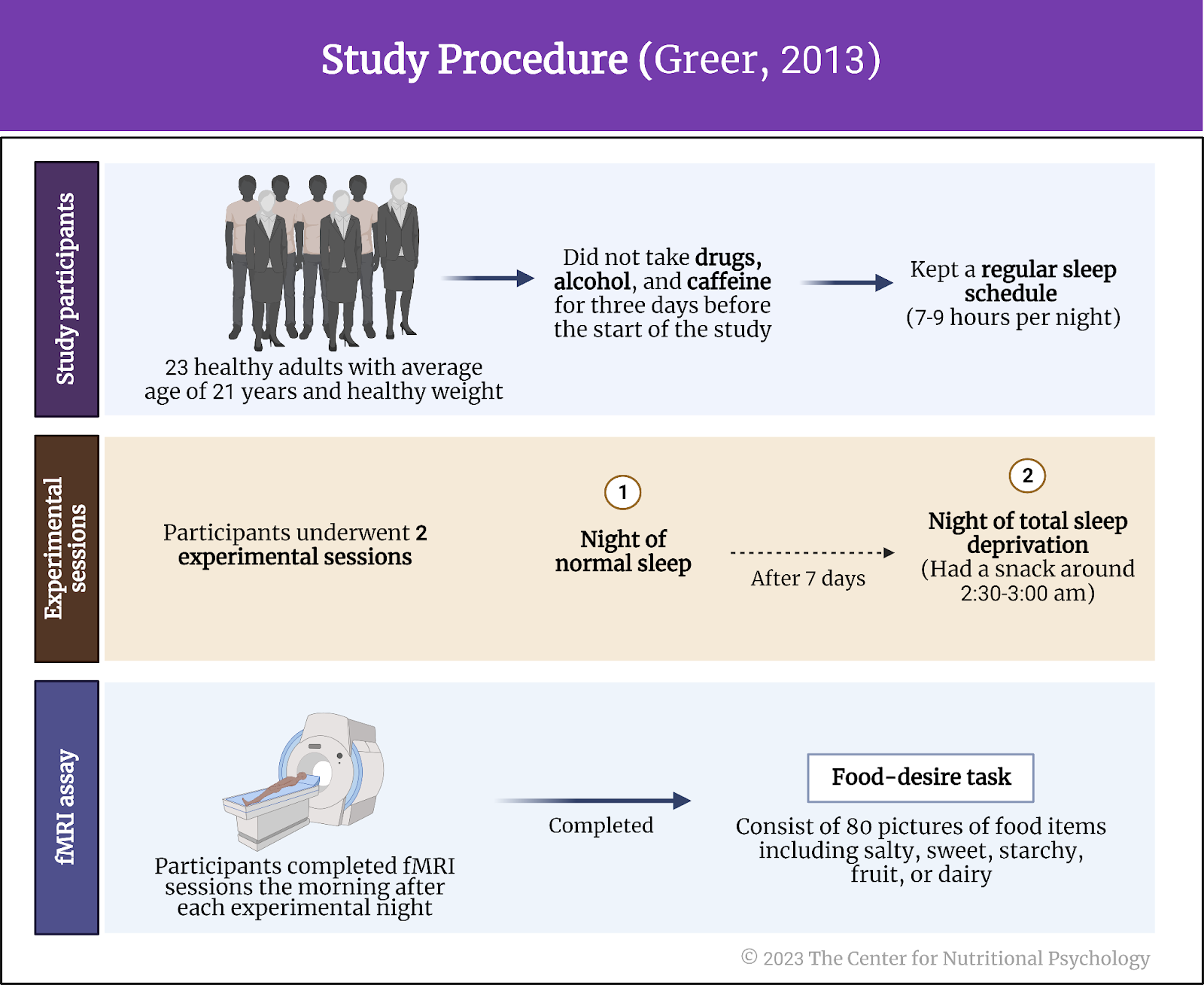
Figure 4. Study procedure (Greer, 2013)
Sleep deprivation diminished activity in the three cortical regions
The activity in the three studied cortex regions was much lower when participants did not sleep (the sleep deprivation night) compared to the night when they slept well. Participants’ overall food desire also increased. The decrease in activity was the most pronounced in the anterior cingulate region.
Activity in the amygdala region increased after the sleepless night
In contrast to the decrease in the cortex activity, the activity in the amygdala brain region in response to desirable food items increased after the sleepless nights when a participant saw a picture of a food item they desired (in the food-desire task taken during brain imaging).
Knowing the function of the amygdala, this increased activity likely indicates increased salience of the food items. After the sleepless night, desirable food items more easily capture participants’ attention. Interestingly, self-reported hunger levels were not different after the two experimental nights (Figure 5).
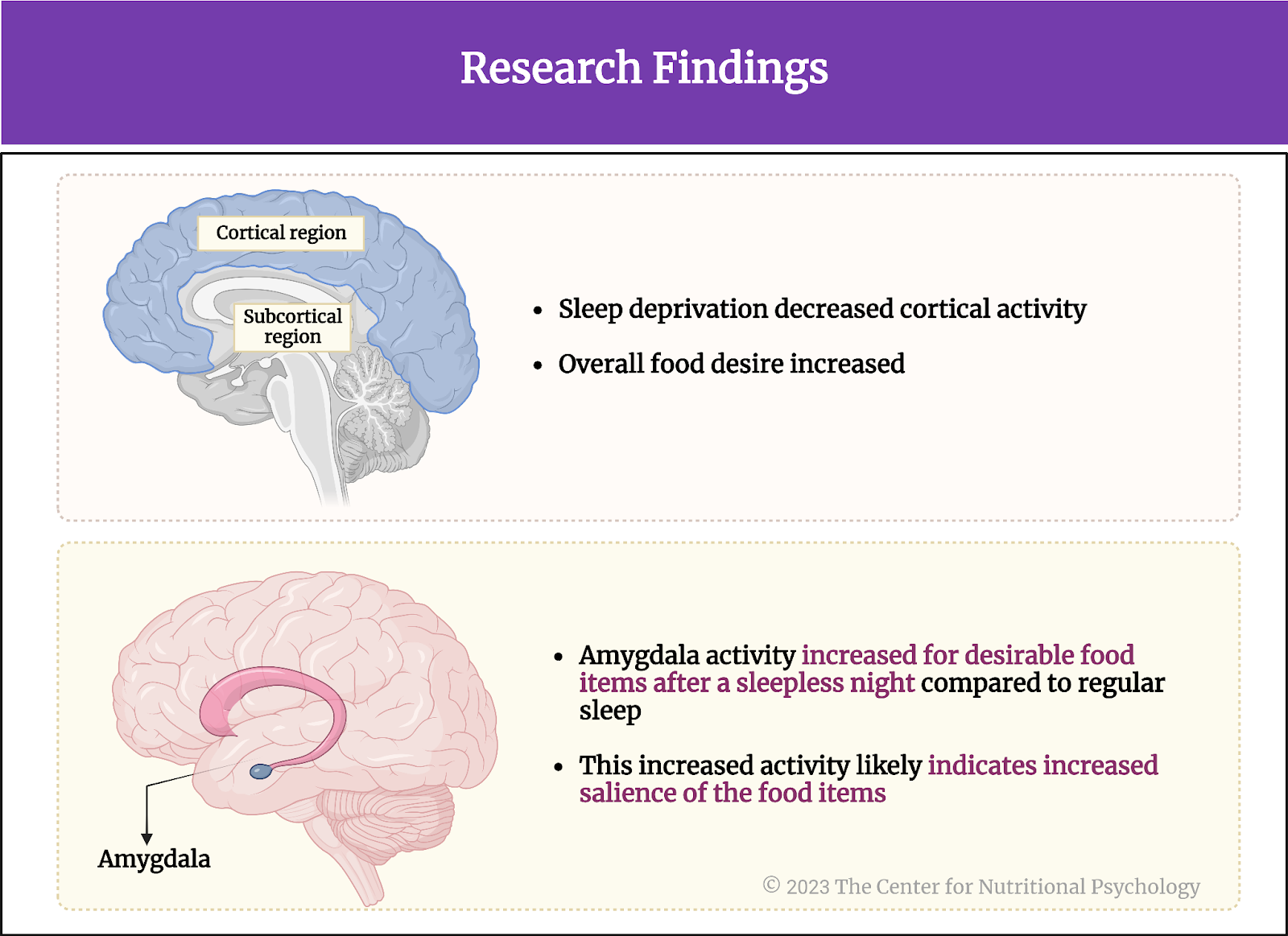
Figure 5. Research Findings
Sleep deprivation increases the desirability of high-calorie food items
After the sleepless night, participants found high-calorie food items much more desirable than the ratings they gave them after the night when they slept normally. However, there were no changes in the desirability of low-calorie items. As a consequence of these changes, the total calorie content of all wanted food items taken together was much higher after the sleepless night. The difference between the food item selections from the two nights was 600 calories on average.
Conclusion
These findings indicate that sleep deprivation blunts the activity of brain regions determining food desirability and choices. While areas responsible for higher-order processes become less reactive to food, those governing our emotional reactions to food increase activity. Consequently, our food-related behaviors become less rational and more emotional when sleep-deprived.
We become more prone to eating tasty food. Since tasty and emotionally pleasing foods also tend to be rich in calories, the calorie intake also increases. The described mechanism explains how sleep loss can lead to the development or maintenance of obesity. Because of this, it is important that treatments for obesity or plans for maintaining a healthy weight also consider this mechanism and include sufficient and undisturbed sleep as one of the factors necessary for maintaining a healthy weight and overall health (see Figure 6).
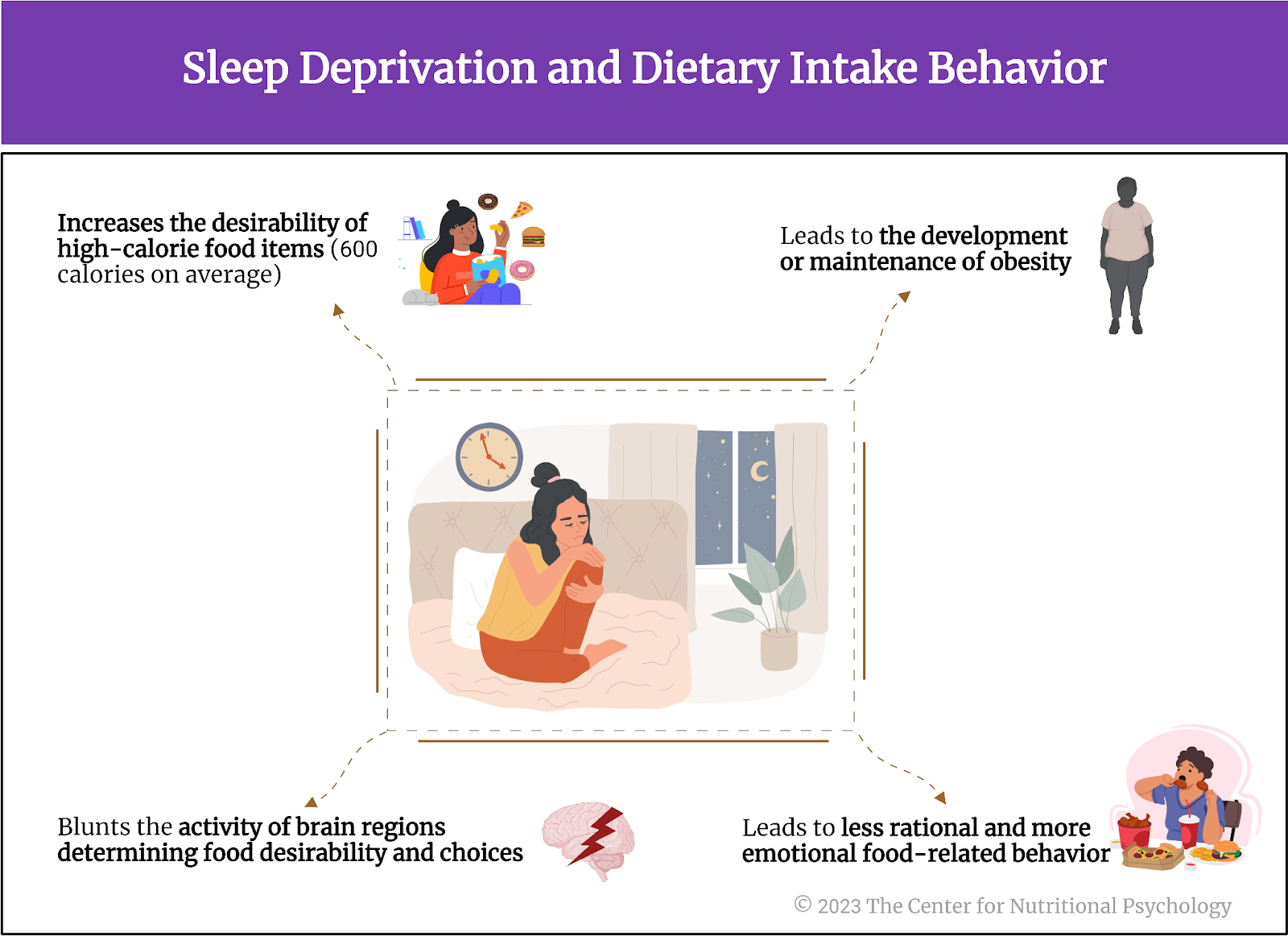
Figure 6. Sleep deprivation and dietary intake behavior
The paper ”The impact of sleep deprivation on food desire in the human brain” was authored by Stephanie M. Greer, Andrea N. Goldstein, and Matthew P. Walker.
Find more studies on the Diet-Sleep Relationship in CNP’s Nutritional Psychology Research Library “Diet, Sleep and Fatigue” Research Category.
References
Bermingham, K. M., May, A., Asnicar, F., Capdevila, J., Leeming, E. R., Franks, P. W., Valdes, A. M., Wolf, J., Hadjigeorgiou, G., Delahanty, L. M., Segata, N., Spector, T. D., & Berry, S. E. (2023). Snack quality and snack timing are associated with cardiometabolic blood markers: the ZOE PREDICT study. European Journal of Nutrition. https://doi.org/10.1007/s00394-023-03241-6
Greer, S. M., Goldstein, A. N., & Walker, M. P. (2013). The impact of sleep deprivation on food desire in the human brain. Nature Communications, 4. https://doi.org/10.1038/ncomms3259
Hillman, D. R., & Lack, L. C. (2013). Public health implications of sleep loss: The community burden. Medical Journal of Australia, 199(8), S7–S10. https://doi.org/10.5694/mja13.10620
Lent, M. R., Atwood, M., Bennett, W. L., Woolf, T. B., Martin, L., Zhao, D., Goheer, A. A., Song, S., McTigue, K. M., Lehmann, H. P., Holzhauer, K., & Coughlin, J. W. (2022). Night eating, weight, and health behaviors in adults participating in the Daily24 study. Eating Behaviors, 45. https://doi.org/10.1016/j.eatbeh.2022.101605
Tzischinsky, O., Latzer, I. T., Alon, S., & Latzer, Y. (2021). Sleep quality and eating disorder-related psychopathologies in patients with night eating syndrome and binge eating disorders. Journal of Clinical Medicine, 10(19). https://doi.org/10.3390/jcm10194613
Wingelaar-Jagt, Y. Q., Bottenheft, C., Riedel, W. J., & Ramaekers, J. G. (2023). Effects of modafinil and caffeine on night-time vigilance of air force crewmembers: A randomized controlled trial. Journal of Psychopharmacology, 37(2), 172–180. https://doi.org/10.1177/02698811221142568








Leave a comment