Can Symptoms of Alzheimer’s be Transferred to Rats via the Gut Microbiota of Alzheimer’s Patients?
- A study published in Brain showed that symptoms of Alzheimer’s disease can be transferred to young rats via the gut microbiota of Alzheimer’s patients
- Transplantation of these microorganisms into the guts of healthy rats induced cognitive deficits
- The deficits resulted from the disruption of adult hippocampal neurogenesis, the capacity of the hippocampus to produce new neurons
Our gut is home to trillions of different microorganisms. These microorganisms sustain themselves using the food we eat and help our digestion process. For example, many food items contain substances called resistant starches. Our digestive system cannot digest these resistant starches, but some bacteria in our gut can. They ferment those types of starches, creating substances called short-chain fatty acids (SCFA) that our bodies can use (Li et al., 2023) (see Figure 1).
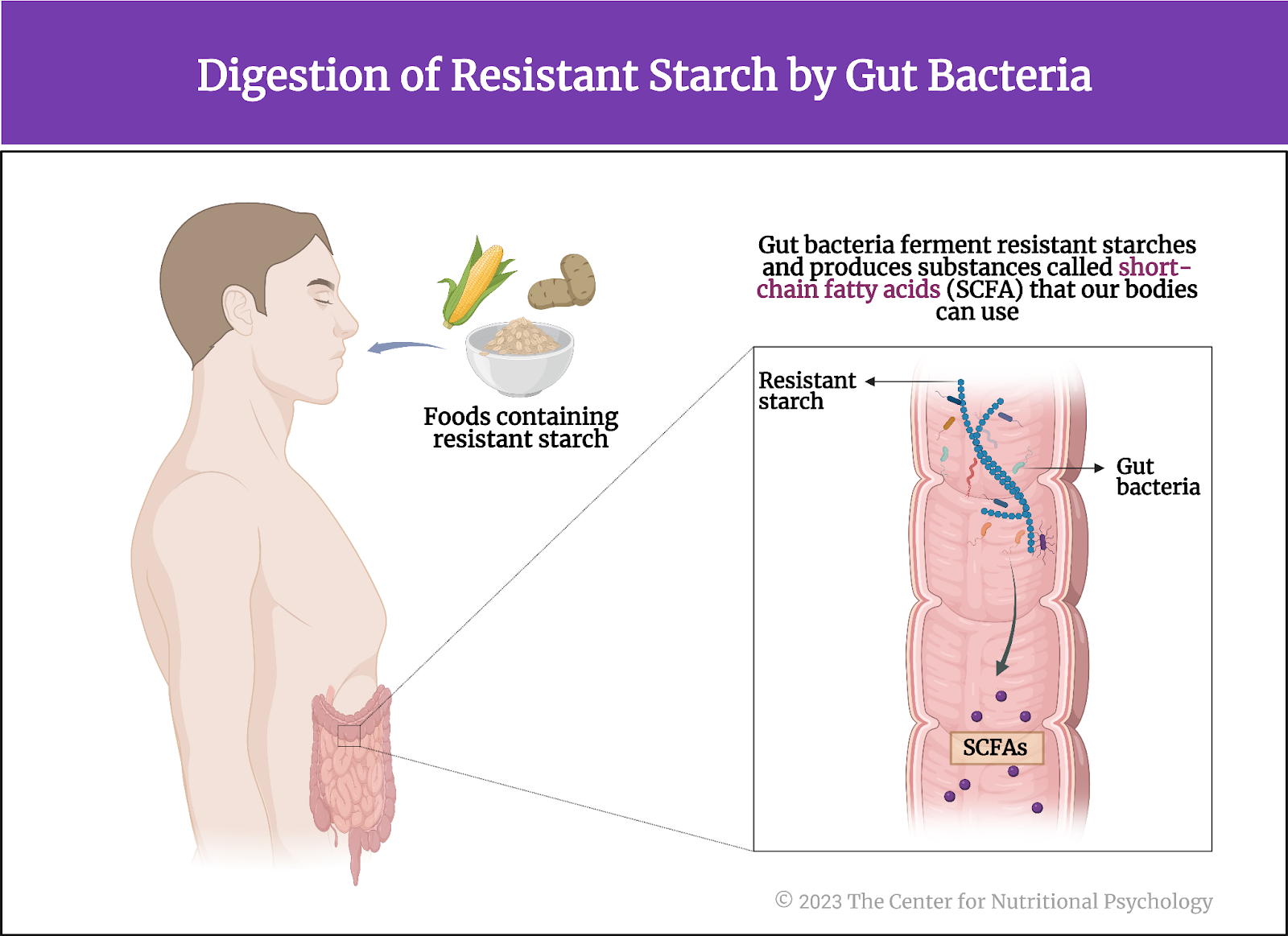
Figure 1. Digestion of resistant starch by gut bacteria
Similarly, these bacteria help digest substances like dietary fiber, other complex polysaccharides, lignans, and many others, converting them into substances our body can use as nutrients or that convey various health benefits. However, when pathogenic, unhealthy microorganisms infect our digestive tract, we experience an upset stomach. This includes symptoms such as nausea, vomiting, diarrhea, abdominal pain, and others.
The microbiota-gut-brain axis (MGBA)
This community of microorganisms living in our gut is called the gut microbiota. However, its effects on us go far beyond helping digestion. Gut microbiota can also influence our central nervous system –and be influenced by it. The mechanism through which this bidirectional communication link between gut microbiota and the brain is achieved is called the microbiota-gut-brain axis (MGBA). It is crucial in regulating various physiological and psychological processes (Carbia et al., 2023; García-Cabrerizo et al., 2021).
The Microbiota-Gut-Brain Axis is crucial in regulating various physiological and psychological processes
This bidirectional communication mechanism occurs via several biomolecules, including the hormone cortisol, short-chain fatty acids (SCFAs), and tryptophan. Emerging studies reveal that the gut microbiota produces substances (called ‘signaling molecules’) that can influence the brain’s activity and responses to stress and emotions (more about this can be found in NP 120 Parts I & II). The MGBA is closely tied to the immune system, influencing the body’s inflammatory responses and potentially contributing to neuroinflammation (Zhu et al., 2023) (see Figure 2).
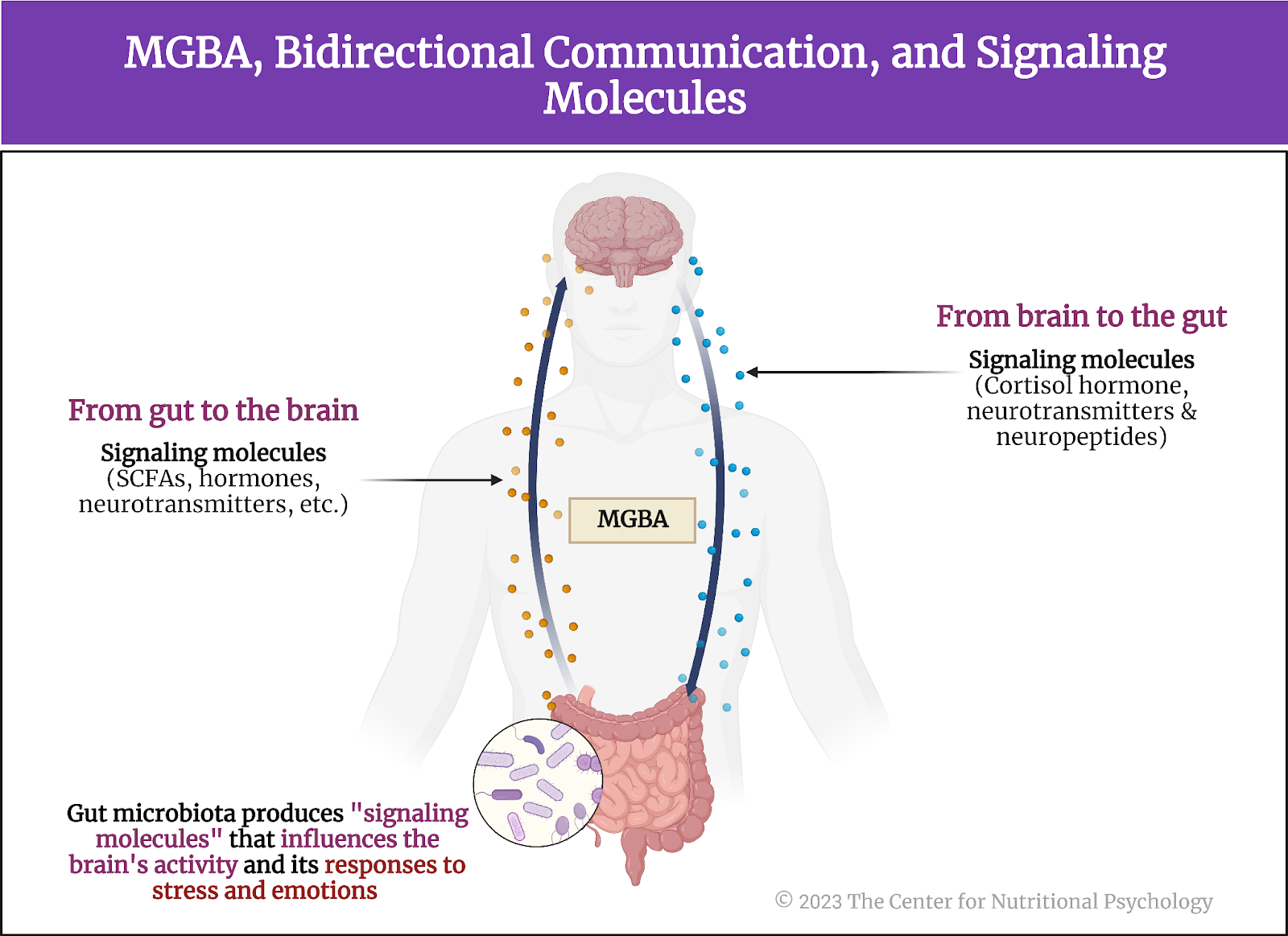
Figure 2. MGBA, bidirectional communication, and signaling molecules
What is Alzheimer’s disease?
Alzheimer’s disease is a progressive neurodegenerative disorder characterized by a decline in cognitive function, memory loss, and changes in behavior. It is the most common form of dementia, affecting primarily older adults. The exact cause of Alzheimer’s disease is not fully understood. Still, it is associated with the accumulation of abnormal protein deposits in the brain, called beta-amyloid plaques and tau tangles. These deposits disrupt communication between nerve cells and eventually lead to their degeneration and death (Grabrucker et al., 2023) (see Figure 3).
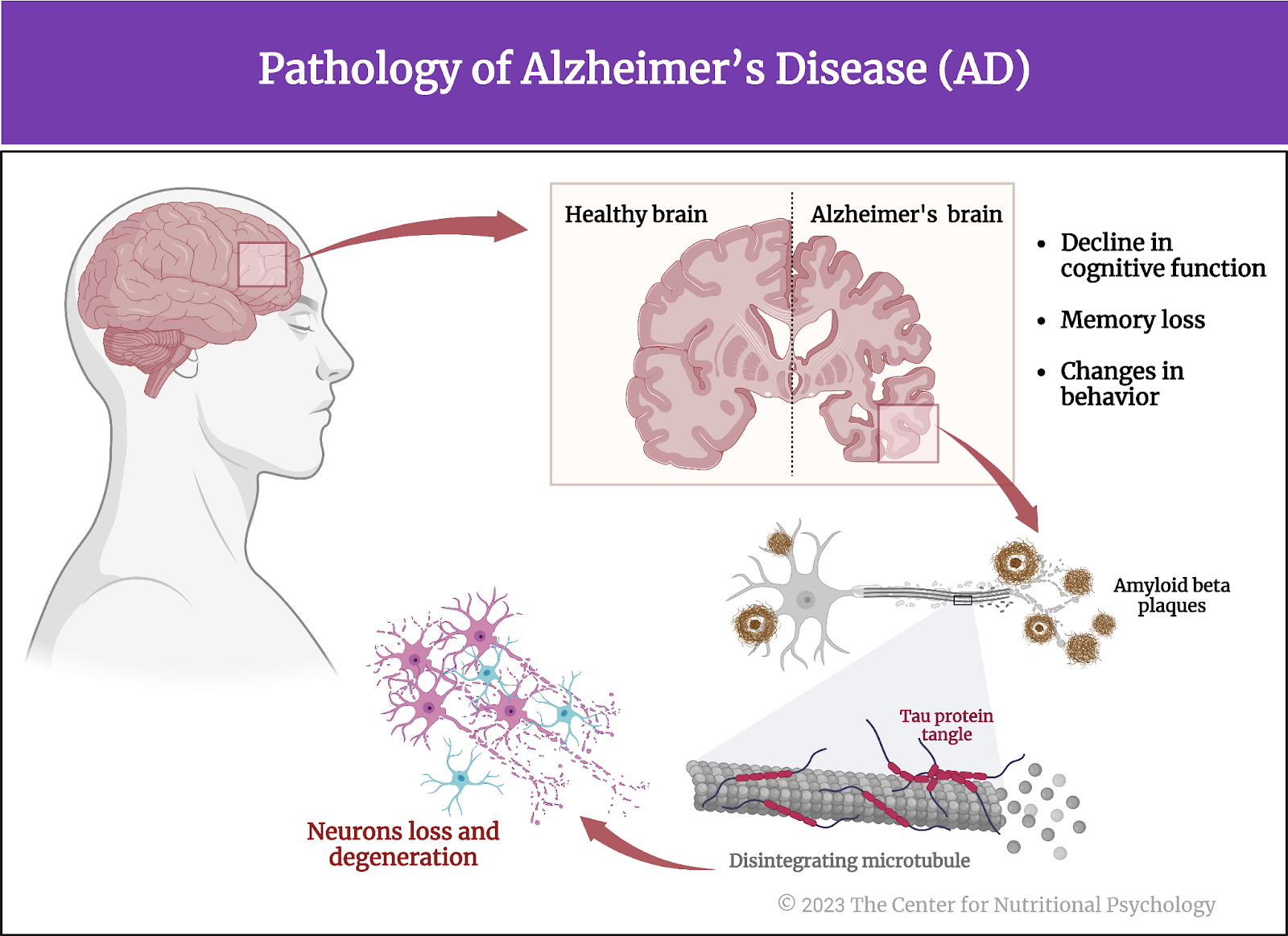
Figure 3. Alzheimer’s Disease (AD)
Alzheimer’s disease and adult hippocampal neurogenesis
The hippocampus is a brain area that is particularly vulnerable to Alzheimer’s disease. It plays a crucial role in learning, memory, and emotional regulation. The hippocampus hosts a population of neural stem cells, special undifferentiated cells that can produce new neurons throughout their lifespan. This process of creating new neural cells is called adult hippocampal neurogenesis. It is crucial for cognitive processes like spatial learning, distinguishing between similar events and environments, and emotion regulation (see Figure 4).
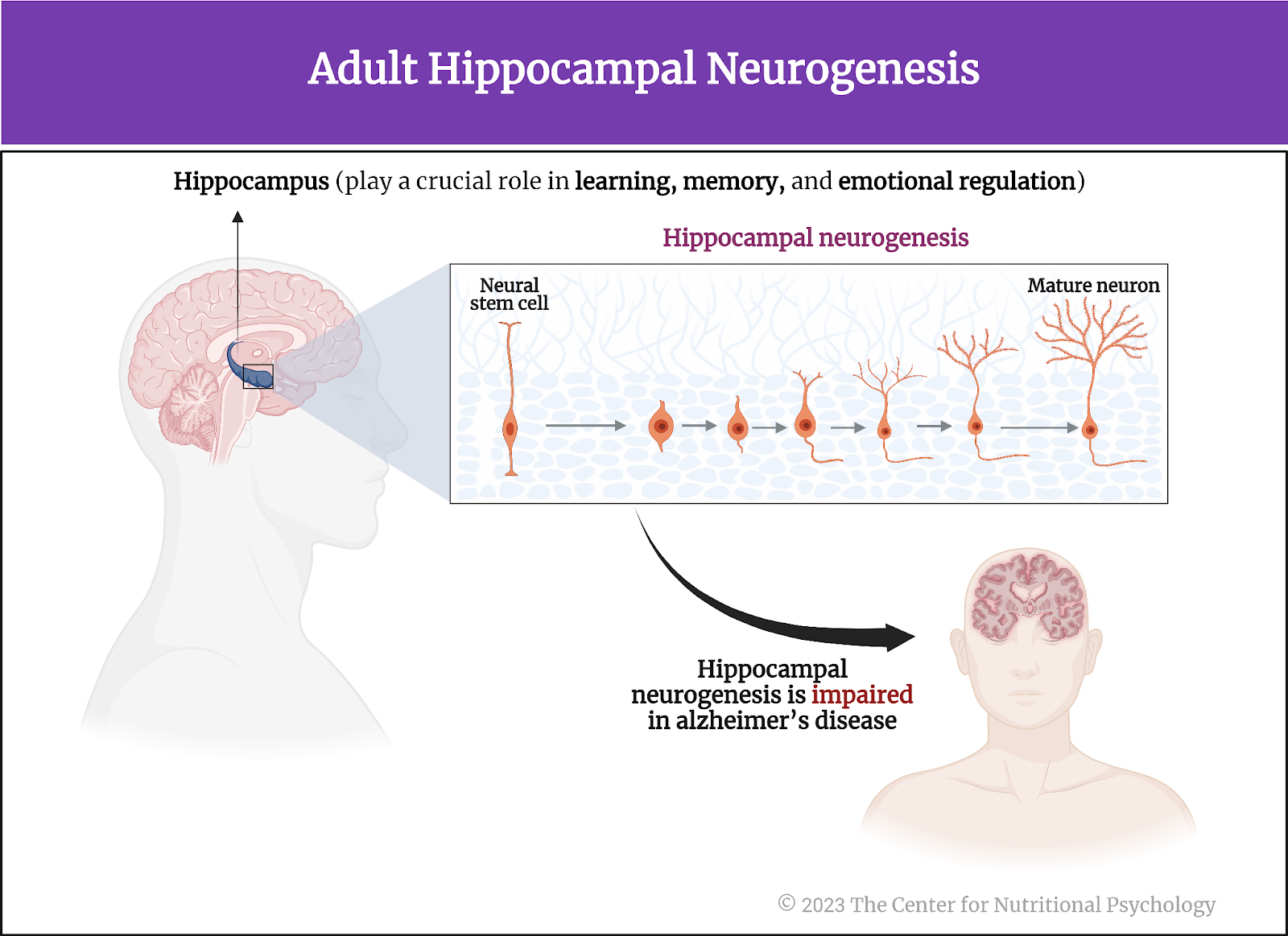
Figure 4. Adult hippocampal neurogenesis
Interestingly, hippocampal neurogenesis is impaired in Alzheimer’s even before abnormal protein deposits can be detected in the brain, including in the hippocampus. This indicates that dysfunction of this system is an early indicator that Alzheimer’s disease is developing (Grabrucker et al., 2023).
The hippocampus is a brain area that is particularly vulnerable to Alzheimer’s disease. It plays a crucial role in learning, memory, and emotional regulation
Causes of Alzheimer’s
Genetic factors, such as specific gene mutations, are linked to early-onset Alzheimer’s, while other risk factors include age, family history, and certain lifestyle factors. Despite ongoing research, there is currently no cure for Alzheimer’s disease, and treatment focuses on managing symptoms and improving the quality of life for affected individuals.
However, the recent discovery of the MGBA has opened a new avenue of research demonstrating that this communication pathway is a significant mediator of behavior throughout the lifespan. Studies indicate clear links between gut microbiota composition and behavior (e.g., Leclercq et al., 2020; Valles-Colomer et al., 2019).
Studies indicate clear links between gut microbiota composition and behavior
Recently, links between the MGBA and Alzheimer’s have begun to appear. Studies on mice indicate that transplanting gut microbiota from Alzheimer’s patients into mice can cause adverse cognitive changes in these mice (Kim et al., 2021; Wang et al., 2022) (see Figure 5). But could it also disrupt the adult hippocampal neurogenesis? And would these changes correlate with the level of cognitive impairment of the person the transplanted microbiota came from?
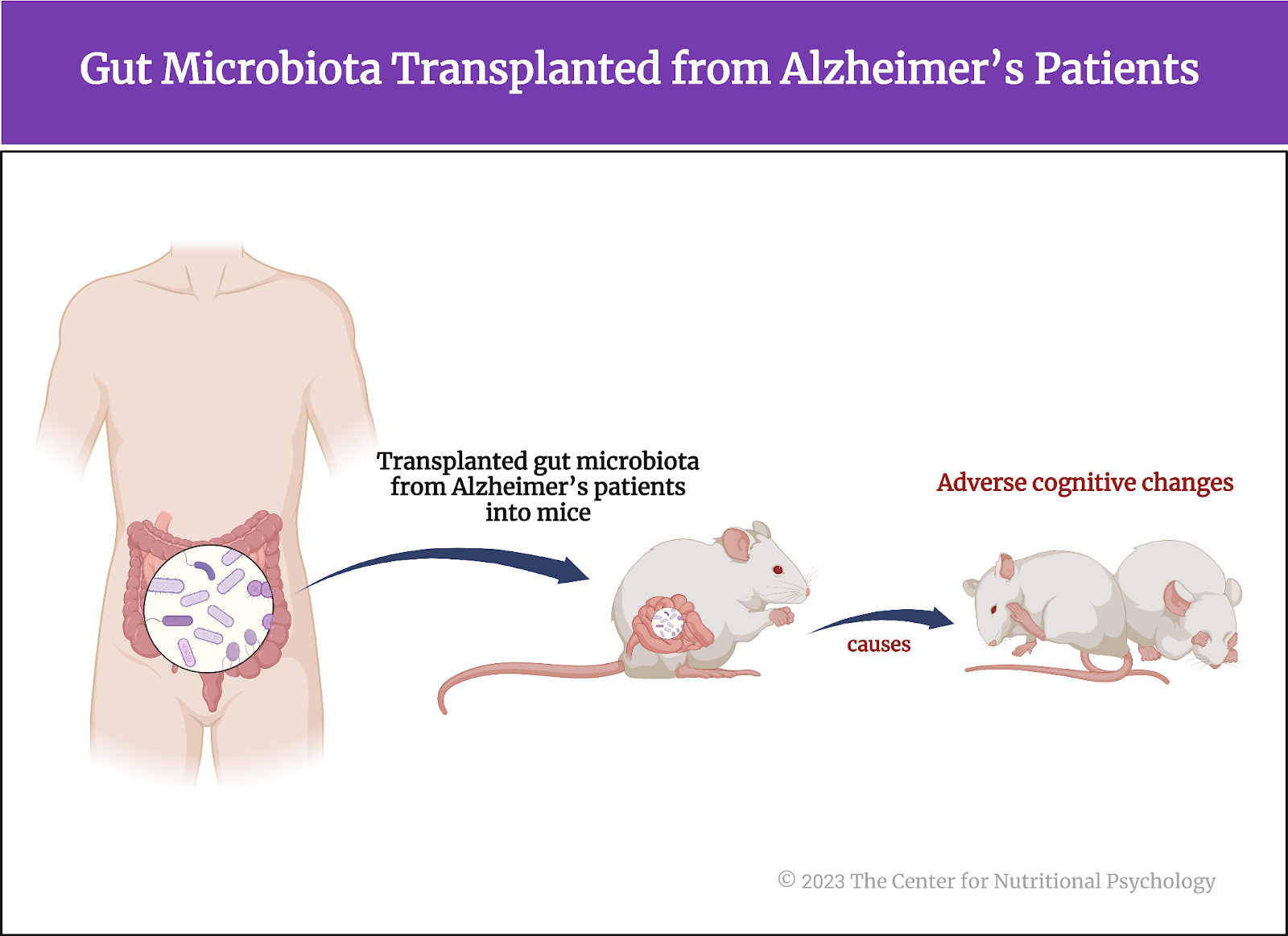
Figure 5. Gut microbiota transplanted from Alzheimer’s patients
The current study
Current studies clearly implicate the gut microbiota in the pathological features of Alzheimer’s disease, but until this particular study, it has remained unclear whether cognitive symptoms in human Alzheimer’s patients and underlying cellular changes (such as the disruption of adult hippocampal neurogenesis) could be transmitted to a healthy organism via the gut microbiota. Study author Stefanie Grabrucker and her colleagues wanted to find out. They also wanted to uncover the mechanism through which this happens.
These authors conducted a study in which they took fecal samples containing gut microbiota from humans suffering from Alzheimer’s disease and transplanted them into the guts of young adult male rats.
Current studies implicate gut microbiota in the pathological features of AD, but until now, it has remained unclear whether cognitive symptoms and underlying cellular changes in human Alzheimer’s patients could be transmitted to a healthy organism via the gut microbiota
The study procedure
Study participants were 69 patients with Alzheimer’s disease, and 64 healthy individuals were included as controls. They were recruited at the IRCCS Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy. They all underwent clinical assessment for cognitive function and a physical examination. All participants gave blood samples for further analysis. Fifty-four participants with Alzheimer’s disease and 41 healthy participants gave stool samples in a sterile cup at their homes. Researchers processed these samples for transplanting gut microbiota found in feces into rats.
Animals used in the study were male Sprague-Dawley rats aged 11 weeks. They were kept in environmentally controlled conditions at a temperature of 21oC and under a 12-hour light-dark cycle (12 hours in light and 12 hours in the dark).
Transplanting gut microbiota from stool samples into rats
Researchers kept rats for two weeks after arrival at the laboratory without any treatment to let them acclimatize. After this period, they added a powerful cocktail of antibiotics to their water for seven consecutive days. This combination of antibiotics consisted of ampicillin (1 g/l), vancomycin (500 mg/l), ciprofloxacin HCL (200 mg/l), and imipenem (250 mg/l). This treatment aimed to deplete rats’ own microbiota so that their digestive system could readily accept the microorganisms to be transplanted from human participants.
After the antibiotic treatment, the study authors randomly allocated the rats into two groups. one was to receive microbiota transplants from Alzheimer’s patients, while the other would receive it from healthy control participants.
They then applied oral gavage of homogenized fecal slurry of human participants (from stool samples) on them for three days. Gavage is a force-feeding method involving inserting a tube into the animal’s esophagus and delivering a measured amount of food directly into the stomach. In this way, study authors transplanted the gut microbiota of human study participants into the guts of these rats (see Figure 6).
Behavior testing and other analyses
Ten days after transplanting the gut microbiota to rats, these researchers conducted a series of behavioral tests on the rats. They conducted the tests during the day (i.e., light cycle), between 9:00 and 19:00. There was a minimum interval of 36 hours between behavioral tests. The tests used were Open Field, Elevated Plus Maze, Modified Spontaneous Location Recognition Test, Novel Object Recognition, Novel Location Recognition, Morris Water Maze, and Forced Swim Test. Their goal was to examine the rats’ cognitive capacity.
Study authors also collected and analyzed fecal samples of the rats during the study. They took blood from their tails ten days after microbiota transplantation for analysis. At the end of the study, the rats were killed, and additional analyses were done on their brain tissue and blood from the trunk (see Figure 6).
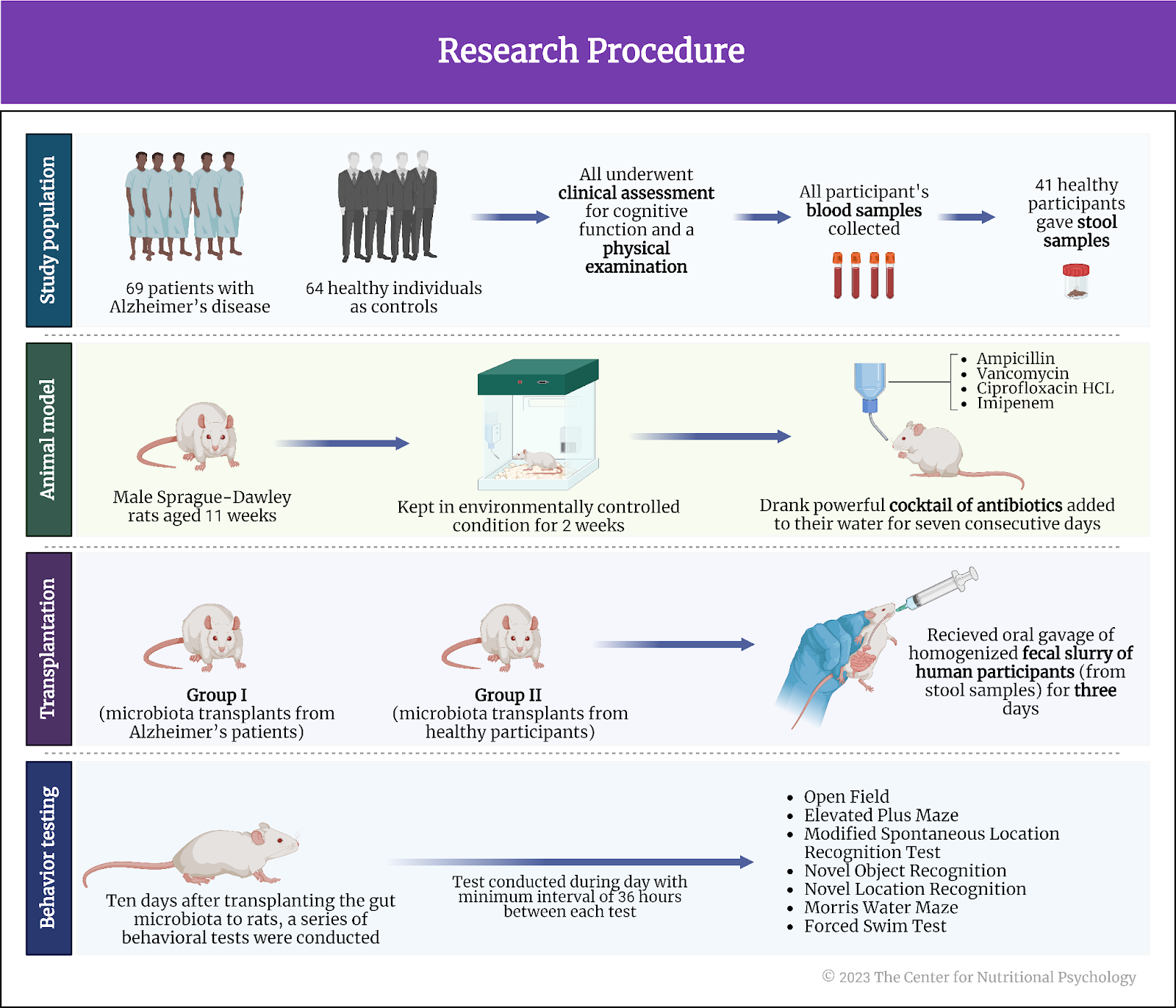
Figure 6. Research procedure
Microbiota composition differed between Alzheimer’s patients and controls
Researchers used metagenomics (bacterial 16S rRNA gene sequencing) on participants’ stool samples to estimate the composition of participants’ gut microbiota. Results revealed no differences in microbiota diversity between participants with and without Alzheimer’s.
However, there were differences in the abundances of specific groups of bacterial species. Alzheimer’s patients had a higher abundance of Bacteroides (particularly of various species associated with inflammatory processes) and Desulfovibrio genera of bacteria. They had lower abundances of Clostridium sensu stricto 1 and the short-chain fatty acid butyrate-producing genera Coprococcus.
Further analyses revealed that participants with Alzheimer’s who had better cognitive function and higher mental clarity (assessed using the Mini-Mental State Examination) tended to have higher abundances of Coproccocus bacteria. These individuals tended to have lower abundances of Desulfovibrio and Dialister species of bacteria.
Gut microbiota from Alzheimer’s patients induced cognitive deficits in rats
Analyses of rat stool samples indicated that gut microbiota transplantation was successful. Comparing rats with microbiota from healthy human participants and those from Alzheimer’s patients, study authors noted that the fecal matter of rats with microbiota from Alzheimer’s patients had higher water content. These rats also increased their water intake, and their colon length decreased. There were other structural changes in the colons of these rats as well.
Behavioral tests showed no changes in rats that received microbiota transplants from healthy human participants. On the other hand, rats with microbiota from Alzheimer’s patients showed impaired ability to recognize familiar locations. They also showed impairment in tasks that relied on different types of memory. Study authors note that all of these cognitive functions depend on the normal functioning of hippocampal neurogenesis.
Rats with microbiota from Alzheimer’s patients showed an impaired ability to recognize familiar locations
Hippocampal neurogenesis was reduced in rats with microbiota from Alzheimer’s patients
Direct assessment of hippocampal neurogenesis in rats that received gut microbiota from Alzheimer’s patients showed that hippocampal neurogenesis was indeed disrupted, confirming the study authors’ suspicions. These rats had substantially fewer new neurons than those that received microbiota from healthy human participants.
Serum of patients with Alzheimer’s reduced the capacity of human brain cells to multiply
The study authors then conducted an in vitro experiment on embryonic human hippocampal progenitor cells (undifferentiated cells found in the hippocampus of the brain, obtained from female human fetuses that were medically terminated). After treating these cells with serum (the liquid component of blood that remains after blood coagulation) taken from the two groups of human study participants, researchers noted that the serum from Alzheimer’s patients decreased the capacity of these cells to multiply.
The capacity of these cells to multiply after treatment depended on the human participant serum from which they came. More specifically, the capacity of these cells to multiply tended to be better after treatment with serum from Alzheimer’s patients with better cognitive functions. The prevalence of indicators of neuron development was higher if serum from Alzheimer’s patients with better cognitive function assessments was used.
Conclusion
The study showed that it is possible to transfer symptoms of Alzheimer’s disease to young, healthy rats by using the gut microbiota of humans suffering from Alzheimer’s disease. This transfer induced a number of changes in the digestive tract and disrupted cognitive functions that depend on preserved hippocampal neurogenesis -–the capacity of stem cells in the hippocampus to generate new neurons (see Figure 7).
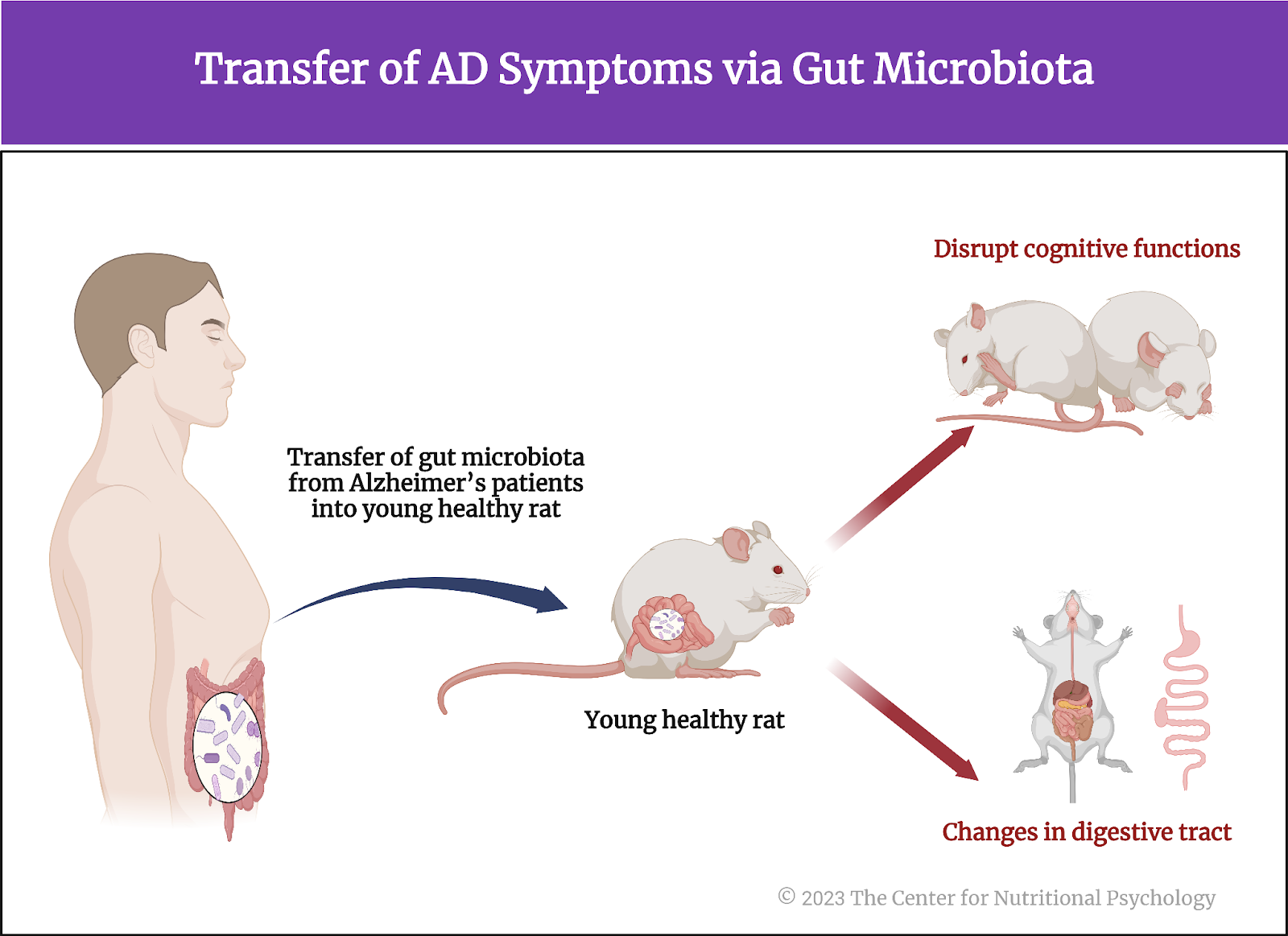
Figure 7. Transfer of AD symptoms via gut microbiota
These results demonstrate that gut microbiota has a causal role in Alzheimer’s disease and that adult hippocampal neurogenesis is central for cognitive impairments in its course. But most importantly, they indicate that Alzheimer’s disease can potentially be transmitted through the fecal-oral route. These findings will likely lead to new ways to prevent and possibly even treat Alzheimer’s disease very soon.
The paper “Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis” was authored by Stefanie Grabrucker, Moira Marizzoni, Edina Silajdžić, Nicola Lopizzo, Elisa Mombelli, Sarah Nicolas, Sebastian Dohm-Hansen, Catia Scassellati, Davide Vito Moretti, Melissa Rosa, Karina Hoffmann, John F. Cryan, Olivia F. O’Leary, Jane A. English, Aonghus Lavelle, Cora O’Neill, Sandrine Thuret, Annamaria Cattaneo, and Yvonne M. Nolan.
References
Carbia, C., Bastiaanssen, T. F. S., Iannone, F., García-cabrerizo, R., Boscaini, S., Berding, K., Strain, C. R., Clarke, G., Stanton, C., Dinan, T. G., & Cryan, J. F. (2023). The Microbiome-Gut-Brain axis regulates social cognition & craving in young binge drinkers. EBioMedicine, 89, 104442. https://doi.org/10.1016/j.ebiom.2023.104442
García-Cabrerizo, R., Carbia, C., O´Riordan, K. J., Schellekens, H., & Cryan, J. F. (2021). Microbiota-gut-brain axis as a regulator of reward processes. Journal of Neurochemistry, 157(5), 1495–1524. https://doi.org/10.1111/JNC.15284
Grabrucker, S., Marizzoni, M., Silajdžić, E., Lopizzo, N., Mombelli, E., Nicolas, S., Dohm-Hansen, S., Scassellati, C., Moretti, D. V., Rosa, M., Hoffmann, K., Cryan, J. F., O’Leary, O. F., English, J. A., Lavelle, A., O’Neill, C., Thuret, S., Cattaneo, A., & Nolan, Y. M. (2023). Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain. https://doi.org/10.1093/brain/awad303
Kim, N., Jeon, S. H., Ju, I. G., Gee, M. S., Do, J., Oh, M. S., & Lee, J. K. (2021). Transplantation of gut microbiota derived from Alzheimer’s disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain, Behavior, and Immunity, 98, 357–365. https://doi.org/10.1016/J.BBI.2021.09.002
Leclercq, S., Le Roy, T., Furgiuele, S., Coste, V., Bindels, L. B., Leyrolle, Q., Neyrinck, A. M., Quoilin, C., Amadieu, C., Petit, G., Dricot, L., Tagliatti, V., Cani, P. D., Verbeke, K., Colet, J. M., Stärkel, P., de Timary, P., & Delzenne, N. M. (2020). Gut Microbiota-Induced Changes in β-Hydroxybutyrate Metabolism Are Linked to Altered Sociability and Depression in Alcohol Use Disorder. Cell Reports, 33(2). https://doi.org/10.1016/J.CELREP.2020.108238
Li, C., Hu, Y., Li, S., Yi, X., Shao, S., Yu, W., & Li, E. (2023). Biological factors controlling starch digestibility in human digestive system. In Food Science and Human Wellness (Vol. 12, Issue 2, pp. 351–358). KeAi Communications Co. https://doi.org/10.1016/j.fshw.2022.07.037
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., Schiweck, C., Kurilshikov, A., Joossens, M., Wijmenga, C., Claes, S., Van Oudenhove, L., Zhernakova, A., Vieira-Silva, S., & Raes, J. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology, 4(4), 623–632. https://doi.org/10.1038/s41564-018-0337-x
Wang, F., Gu, Y., Xu, C., Du, K., Zhao, C., Zhao, Y., & Liu, X. (2022). Transplantation of fecal microbiota from APP/PS1 mice and Alzheimer’s disease patients enhanced endoplasmic reticulum stress in the cerebral cortex of wild-type mice. Frontiers in Aging Neuroscience, 14. https://doi.org/10.3389/fnagi.2022.858130
Zhu, X., Sakamoto, S., Ishii, C., Smith, M. D., Ito, K., Obayashi, M., Unger, L., Hasegawa, Y., Kurokawa, S., Kishimoto, T., Li, H., Hatano, S., Wang, T. H., Yoshikai, Y., Kano, S. ichi, Fukuda, S., Sanada, K., Calabresi, P. A., & Kamiya, A. (2023). Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nature Immunology. https://doi.org/10.1038/s41590-023-01447-8








Leave a comment