Introduction
The past several decades have seen the rise of an obesity pandemic that is ongoing worldwide. While obese individuals were quite rare just a century ago, 2015-2018 estimates for the U.S. state that more than two-thirds of the adult population is overweight or obese (Wong et al., 2022). Determining the causes of this increase in obesity rates has attracted much research attention. Studies have revealed a complex interplay between diet components, environmental factors, and previously unknown psychological and physiological mechanisms resulting in overeating and obesity in the long term. These novel studies on the intersection of nutrition and psychology are part of a developing field of science called nutritional psychology (The Center for Nutritional Psychology, 2023) (see Figure 1).
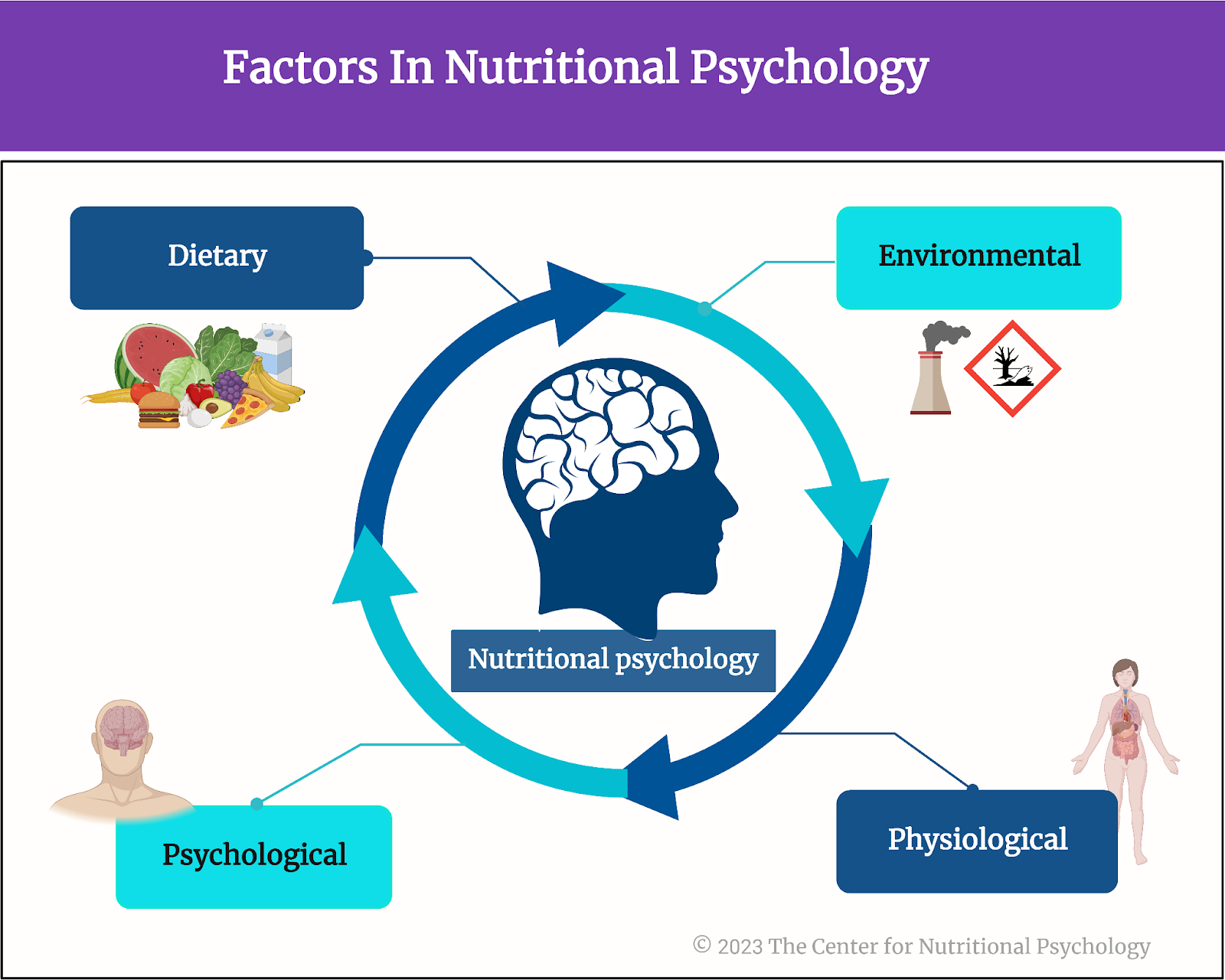
Figure 1. Diet, environment, psychological, and physiological factors in nutritional psychology
There is a complex interplay between diet, environmental factors, and psychological and physiological mechanisms resulting in overeating and obesity
Gut microbiota and the microbiota-gut-brain axis
The human gut microbiome consists of trillions of microorganisms that live in the human intestinal tract. These microorganisms play a key role in digesting the food we eat. However, their influence extends beyond the gut, encompassing crucial roles in metabolic regulation, body weight maintenance, and immune system modulation.
This growing body of evidence suggests that these gut microorganisms also profoundly impact brain functions, mood, cognition, and emotional well-being (Zhu et al., 2023). This topic is explored in continuing education curricula within nutritional psychology — particularly how the gut microbiota and the gut-brain axis interconnect with the diet-mental health relationship to influence psychological functioning and experience, shedding light on its potential therapeutic implications for mental health outcomes.
This growing body of evidence suggests that gut microorganisms profoundly impact brain functions, mood, cognition, and emotional well-being
Scientists have recently discovered a communication pathway connecting the gut microbiome and the brain. This pathway is called the microbiota-gut-brain axis. It is based on small proteins called cytokines and a number of other biomolecules, including the hormone cortisol, short-chain fatty acids (SCFAs), tryptophan, and others.
The Western diet
The Western diet is a modern dietary pattern prevalent in Western societies, characterized by a high intake of processed and hyperpalatable foods with increased contents of fat, sugary snacks, and refined grains. It typically includes low consumption of fruits, vegetables, unprocessed-high-quality proteins, nuts, and seeds. This diet’s excessive reliance on added sugars and unhealthy fats has been linked to an increased risk of obesity, metabolic syndrome, and various chronic diseases.
Studies have indicated that feeding mice a Western diet causes inflammation in the region of the brain called the hypothalamus (Heiss et al., 2021; Thaler et al., 2013). Inflammation of the hypothalamus damages the neurons and leads to the formation of scars made of glial cells. This is called gliosis. Inflammation of the hypothalamus often happens before a mouse starts gaining weight. Due to this, scientists believe it might cause weight gain by causing leptin resistance.
Studies have indicated that feeding mice a Western diet causes inflammation of the region of the brain called the hypothalamus
Leptin and leptin resistance
Leptin is a hormone produced by fat cells during eating. It regulates appetite and body weight and is produced in proportion to the amount of fat in the body. Leptin concentrations inform the brain of how much fat is stored. Increased leptin concentrations (normally caused by an abundance of body fat) “tell” the brain to decrease food intake and increase energy expenditure.
Leptin is a hormone produced by fat cells that regulate appetite and body weight
Factors such as chronic inflammation or eating high-fat diets (HFDs) may cause the body to be less receptive to leptin. This is called leptin resistance. Leptin resistance results in disrupted appetite and energy regulation, i.e., the brain does not reduce food intake in spite of the abundance of body fat. This can contribute to obesity and cause difficulty controlling body weight (Thaler et al., 2013) (see Figure 2).
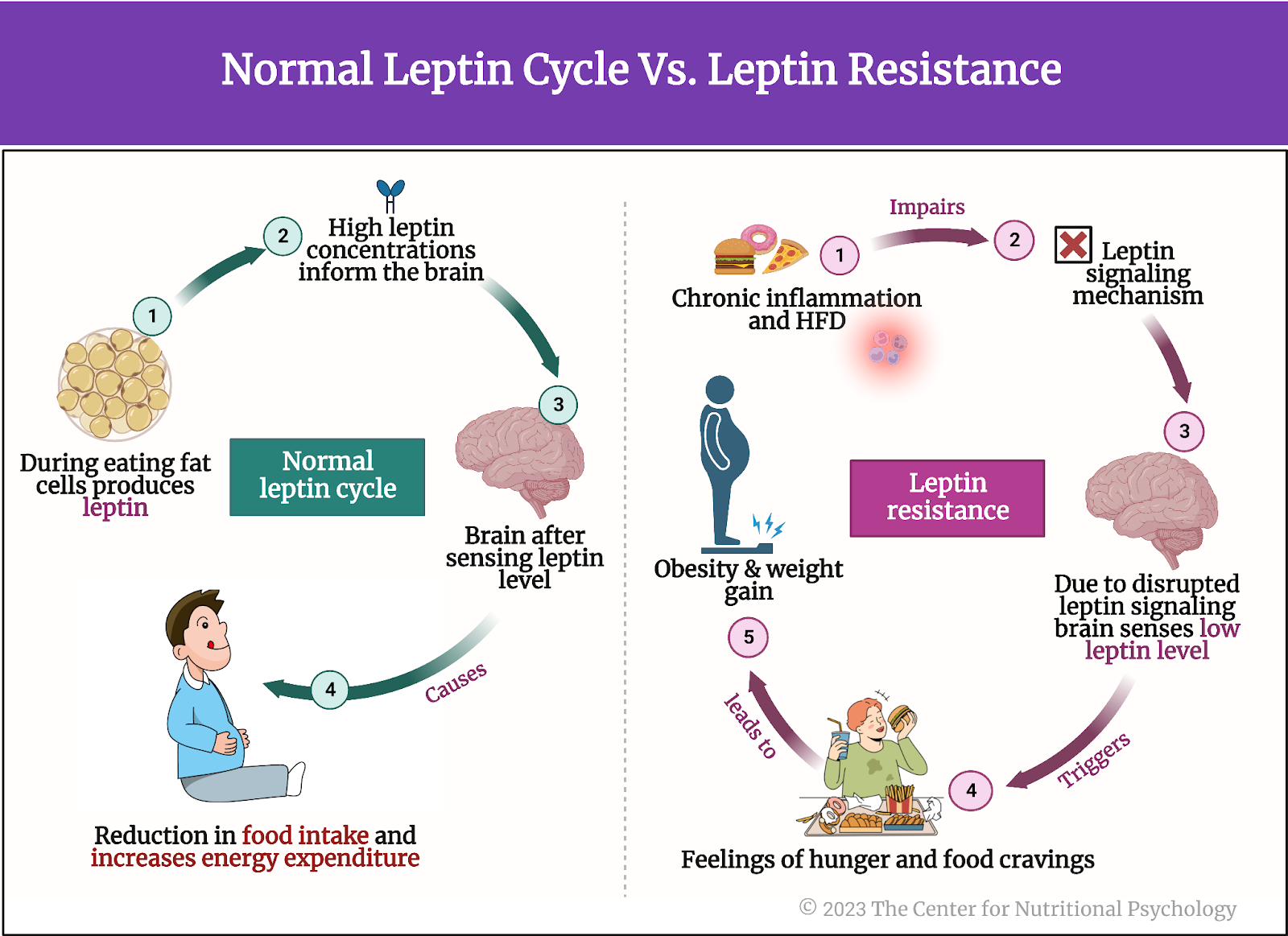
Figure 2. Normal leptin cycle versus leptin resistance.
Gliosis, leptin resistance, and gut microbiota
Microglia are a type of immune cell in the central nervous system that helps protect and maintain the brain and spinal cord by detecting and responding to potential threats or damage. Studies have shown that activation of microglia cells that happens during inflammation of the hypothalamus might be causing leptin resistance. Removing these microglia cells from the hypothalamus has improved sensitivity to leptin. Improved sensitivity to leptin allows the brain to recognize when enough fat is stored in the body and reduce food intake.
Studies have shown that the activation of microglia cells that happens during inflammation of the hypothalamus might be causing leptin resistance
Intriguingly, according to the scientific evidence presented in our recent NP 120 course, it has been discovered that the gut microbiota plays a significant role in regulating the development and maturation of microglia cells and influencing their function. Although the mechanism of this action remains unknown, it has led scientists to believe there might be a link between hypothalamus inflammation and gut microbiota (Heiss et al., 2021) (see Figure 3).
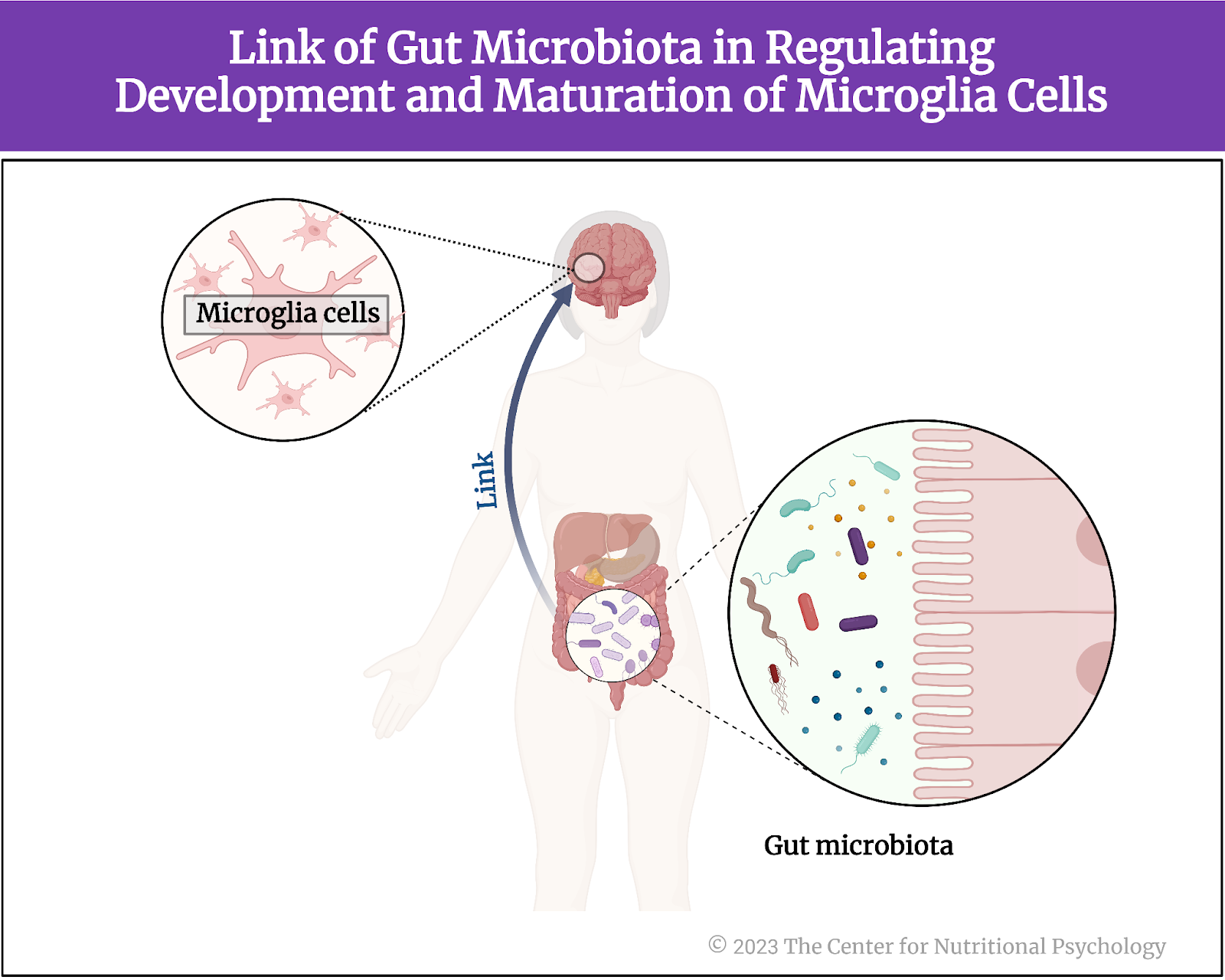
Figure 3. Link of Gut Microbiota in regulating development and maturation of microglia cells
There might be a link between hypothalamus inflammation and gut microbiota (Heiss et al., 2021)
The current study
Study author Christina N. Heiss and her colleagues wanted to know whether mice that lack gut microbiota are protected against diet-induced inflammation of the hypothalamus. They noted previous studies’ results showing mice with depleted gut microbiota and those treated with antibiotics to be protected from diet-induced obesity.
In other words, they noted that mice that consume a Western diet, a diet that normally leads to obesity in mice, do not become obese if microbiota are not present in their guts. This might be because the absence of microbiota prevents inflammation of the hypothalamus, which would, in turn, prevent leptin resistance from developing. If this is the case, the mechanism for preventing overeating based on leptin would remain intact, preventing mice from becoming obese. Alternatively, it could be that, without microbiota, the guts of mice could not digest complex nutrients from the food they eat, thus substantially reducing the amount of nutrition they can derive from food. In this case, obesity would be avoided because their bodies cannot use their food. But which of these is the case?
The procedure
The study authors used three groups of male mice, 10-13 weeks old – conventionally raised mice, germ-free mice, and antibiotic-fed mice. They used several genetic groups of mice, including a strain of genetically engineered mice that allow for controlled and regulated manipulation of specific genes in specific tissues (Tamoxifen-inducible Cre mice).
In the scope of the experiments, researchers fed mice either a chow diet or a Western diet. Western diet was given for either 2 days, 1 week, or 4 weeks, depending on the experiment conducted in the scope of the study.
The chow diet for mice is a nutritionally balanced and standardized diet formulated to provide essential nutrients required for the health and growth of laboratory mice. It typically consists of a combination of proteins, carbohydrates, fats, vitamins, and minerals in pellet or block form. The Western diet used in this study was high in fat and sucrose, with 40% of calories coming from fat.
All food for mice was sterilized, i.e., underwent procedures that killed all microorganisms in the food before mice ate it. The chow diet was sterilized in an autoclave, which uses high pressure and steam to kill microorganisms. The Western diet food was irradiated, i.e., radiation was used to kill microorganisms.
The mice
Conventionally raised mice were laboratory mice kept in regular conditions and fed a normal diet for laboratory mice. They have normal gut microbiota.
Germ-free mice are created through techniques that ensure they do not acquire gut microbiota from birth through their entire lifetimes. They are typically born using cesarean section deliveries of pregnant mice in a sterile environment. This is done to prevent the transfer of microbes during birth. After that, they are kept in specialized sterile isolation spaces called isolators or bubbles that maintain a controlled germ-free environment.
These isolators provide filtered air, sterile food, and autoclaved water to prevent microbial contamination. Researchers raising these mice take special care to maintain strict barrier measures, including specialized clothing and equipment, to prevent the unintentional introduction of microorganisms. They regularly monitor these mice’s bodily fluids and tissues through special techniques to ensure the absence of any detectable microorganisms. Germ-free mice are typically leaner than conventionally raised mice and, consequently, have lower leptin levels in circulation.
Antibiotic-fed mice in this study had 1g of ampicillin and 0.5g of neomycin added per liter of their drinking water. Ampicillin and neomycin are antibiotics. Researchers kept the drinking water with antibiotics added in bottles protected from light. They prepared a new solution every second day. Researchers started giving this water with antibiotics to mice three days before changing their regular diet to a Western diet.
Mice without gut microbiota are protected from diet-induced inflammation of the hypothalamus
Results showed that conventionally raised mice fed a Western diet for 1 week developed gliosis in the hypothalamus. Inflammation indicators were increased in these mice in the part of the hypothalamus called the arcuate nucleus compared to conventionally raised mice fed regular mice food (chow) (see Figure 4).
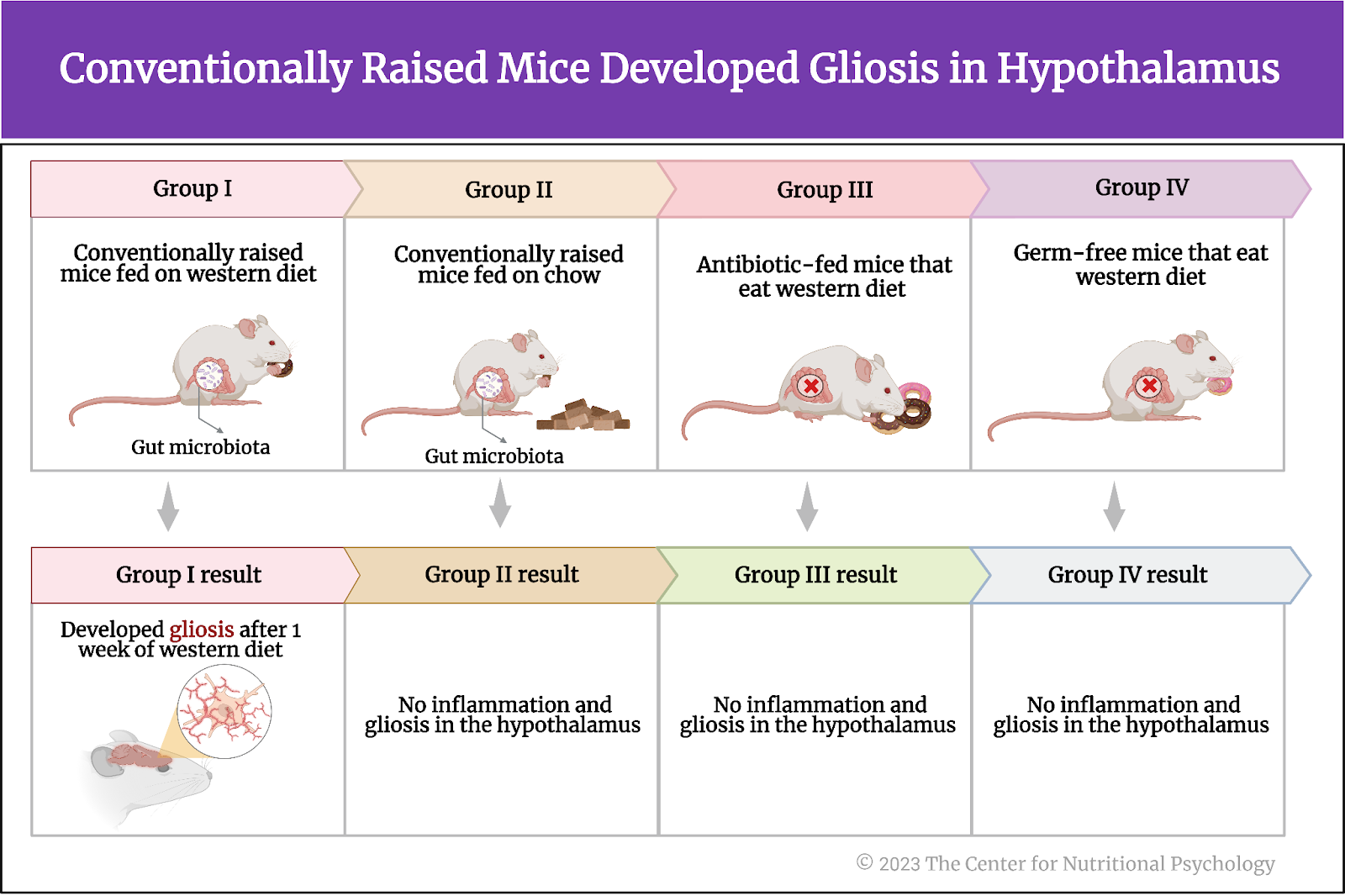
Figure 4. Conventionally raised mice fed a Western diet developed gliosis in the hypothalamus
When researchers examined germ-free mice and mice whose gut microbiota were depleted using antibiotic treatment (antibiotic-fed mice), results showed that these mice also showed no increase in inflammation indicators or the proliferation of microglia cells after eating a Western diet for a week.
Gliosis in the hypothalamus leads to greater gain in body weight and fat mass
The study authors also wanted to know whether gliosis in the hypothalamus caused by a Western diet leads, in turn, to increased body weight and fat mass accumulation in mice. To test that, they fed conventionally raised mice and antibiotic-fed mice a Western diet for 4 weeks.
Results showed that conventionally raised mice fed a Western diet gained more body weight and fat mass than antibiotic-fed mice. Compared to antibiotic-fed mice, they also had increased hypothalamus inflammation indicators and increased numbers of a specific type of microglia cells (iba1-positive microglia).
There was no association between the number of microglia in the arcuate nucleus region of the hypothalamus and changes in body weight or fat mass at the end of the 4 weeks. However, fat mass and the relative increase in fat mass during the study were associated with the number of a specific type of glial cells called astrocytes.
Further analysis showed that germ-free and antibiotic-fed mice are more sensitive to leptin than conventionally raised mice. When researchers gave them leptin injections, the first two types of mice reduced their food intake more than conventionally raised mice did.
Glucagon-like-peptide 1 (GLP-1) seems to be crucial for protection against diet-induced inflammation of the hypothalamus
Germ-free and antibiotic-fed mice had higher levels of the hormone called glucagon-like peptide 1 (GLP-1) when they were fed a regular diet. This hormone secreted in the small intestine’s intestinal lining cells (L cells) is important in regulating blood sugar levels. It also helps reduce inflammation and protect neurons.
Study authors believed it might also be crucial for the protection from inflammation of the hypothalamus induced by the Western diet. After mice were fed a Western diet for a week, antibiotic-treated and germ-free mice had higher levels of GLP-1 than conventionally raised mice. These mice did not gain weight or develop hypothalamic inflammation after this diet. However, when researchers measured these same things in antibiotic-fed and germ-free mice whose GLP-1 signaling pathway was disabled, they also gained weight and developed inflammation, similar to conventionally raised mice. This indicated that the functional signaling path of GLP-1 is crucial for countering the inflammation of the hypothalamus induced by a Western diet.
Just a week on a Western diet led to inflammation of the hypothalamus that, in turn, disrupted the body’s mechanism for regulating food intake
Conclusion
These findings in mice show that gut microbiota changes how the organism, of mice in this case, reacts to a Western diet. When gut microbiota was intact, just a week on a Western diet led to inflammation of the hypothalamus that, in turn, disrupted the body’s mechanism for regulating food intake. However, when gut microbiota was depleted or absent, this inflammation did not happen, provided that the signaling pathway of one specific hormone (GLP-1) was intact.
While the study was done on mice, similar physiological mechanisms exist in humans. Due to this, these findings on mice help scientists better understand how and through which physiological mechanisms changes in the human diet that occurred in the last century disrupted food intake regulation in the human body leading to the current obesity pandemic.
The paper “The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism” was authored by Christina N. Heiss, Louise Manneras-Holm, Ying Shiuan Lee, Julia Serrano-Lobo, Anna Hakansson Gladh, Randy J. Seeley, Daniel J. Drucker, Fredrik Backhed, and Louise E. Olofsson.
References
Heiss, C. N., Mannerås-Holm, L., Lee, Y. S., Serrano-Lobo, J., Håkansson Gladh, A., Seeley, R. J., Drucker, D. J., Bäckhed, F., & Olofsson, L. E. (2021). The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Reports, 35(8). https://doi.org/10.1016/j.celrep.2021.109163
Thaler, J. P., Guyenet, S. J., Dorfman, M. D., Wisse, B. E., & Schwartz, M. W. (2013). Hypothalamic inflammation: Marker or mechanism of obesity pathogenesis? Diabetes, 62(8), 2629–2634. https://doi.org/10.2337/DB12-1605
The Center for Nutritional Psychology. (2023). What is Nutritional Psychology? https://www.nutritional-psychology.org/what-is-nutritional-psychology/
Wong, M. C., Mccarthy, C., Fearnbach, N., Yang, S., Shepherd, J., & Heymsfield, S. B. (2022). Emergence of the obesity epidemic: 6-decade visualization with humanoid avatars. The American Journal of Clinical Nutrition, 115(4), 1189–1193. https://doi.org/10.1093/AJCN/NQAC005
Zhu, X., Sakamoto, S., Ishii, C., Smith, M. D., Ito, K., Obayashi, M., Unger, L., Hasegawa, Y., Kurokawa, S., Kishimoto, T., Li, H., Hatano, S., Wang, T. H., Yoshikai, Y., Kano, S. ichi, Fukuda, S., Sanada, K., Calabresi, P. A., & Kamiya, A. (2023). Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nature Immunology. https://doi.org/10.1038/s41590-023-01447-8