Researchers Identify Neural Pathways Transmitting Anti-Inflammatory Effects of Hunger

Listen to this Article
- A series of experiments on mice published in Cell Reports found that hunger reduces inflammatory responses after an injury.
- The anti-inflammatory effects of hunger were more robust than those of non-steroidal anti-inflammatory drugs.
- AgRP neurons projecting to the paraventricular nucleus of the brain’s hypothalamus region mediate the majority of hunger’s anti-inflammatory effects.
People need food to survive. Severe malnutrition causes the body to break down its own tissues to meet energy needs. The body first utilizes the stored fat, but if starvation continues, it eventually uses muscle mass to fulfill its nutritional needs. Prolonged starvation impairs vital functions and leads to organ failure, weakened immune response, and severe hormonal imbalances (Sidiropoulos, 2007). Cognitive functions also deteriorate. But what happens when people restrict their food intake only for limited periods?
Restricted food intake
While the consequences of a chronic lack of food are dire, cultures worldwide have long believed that restricted food intake for limited periods of time can produce beneficial psychological and physiological consequences.
The practice of fasting, or abstaining from all or some kinds of food or drink for a specific period of time, is an important part of almost all major religions. Religions practice fasting as a means of spiritual discipline, self-reflection, and expressing devotion or penitence. It is often seen as a way to cleanse the body and mind, allowing for deeper religious or spiritual contemplation and connection with the divine or higher power.
Cultures worldwide have long believed that restricted food intake for limited periods of time can produce beneficial psychological and physiological consequences
Research studies show that restricted food intake for a limited period leads to complex physiological changes in the central and peripheral nervous systems. These changes can inhibit inflammation by preventing the activation of inflammasomes, multi-protein complexes that activate an inflammatory response. Additionally, restricted food intake reduces the production and release of pro-inflammatory cytokines, a group of signaling proteins secreted by immune cells that promote inflammation (Klima et al., 2023). On the psychological side, however, restrained eating can increase the feeling of hunger and food craving (Dicker-Oren et al., 2022).
Restricted food intake for a limited period can inhibit inflammation by preventing the activation of inflammasomes, multi-protein complexes that activate an inflammatory response, and reduce the production and release of pro-inflammatory cytokines
While inflammation is an adaptive response, helping the body fight off infection or facilitating the repair of damaged tissue, long-term inflammation can become maladaptive and impair basic actions necessary for survival. It is also the cause or a contributing factor to many diseases and adverse medical conditions.
On the psychological side, however, restrained eating can increase the feeling of hunger and food craving (Dicker-Oren et al., 2022)
Agouti-related peptide (AgRP) neurons – hunger neurons
An important part of the neural circuits that react to restricted food intake are agouti-related peptide neurons, or AgRP neurons for short. Often referred to as “hunger neurons,” they are a specific type of neuron located in the brain’s arcuate nucleus of the hypothalamus region. These neurons play a critical role in regulating feeding behavior and energy homeostasis. AgRP neurons produce neuropeptides, including agouti-related peptide (AgRP) and neuropeptide Y (NPY), which are potent stimulators of appetite (Sternson & Atasoy, 2014) (see Figure 1).
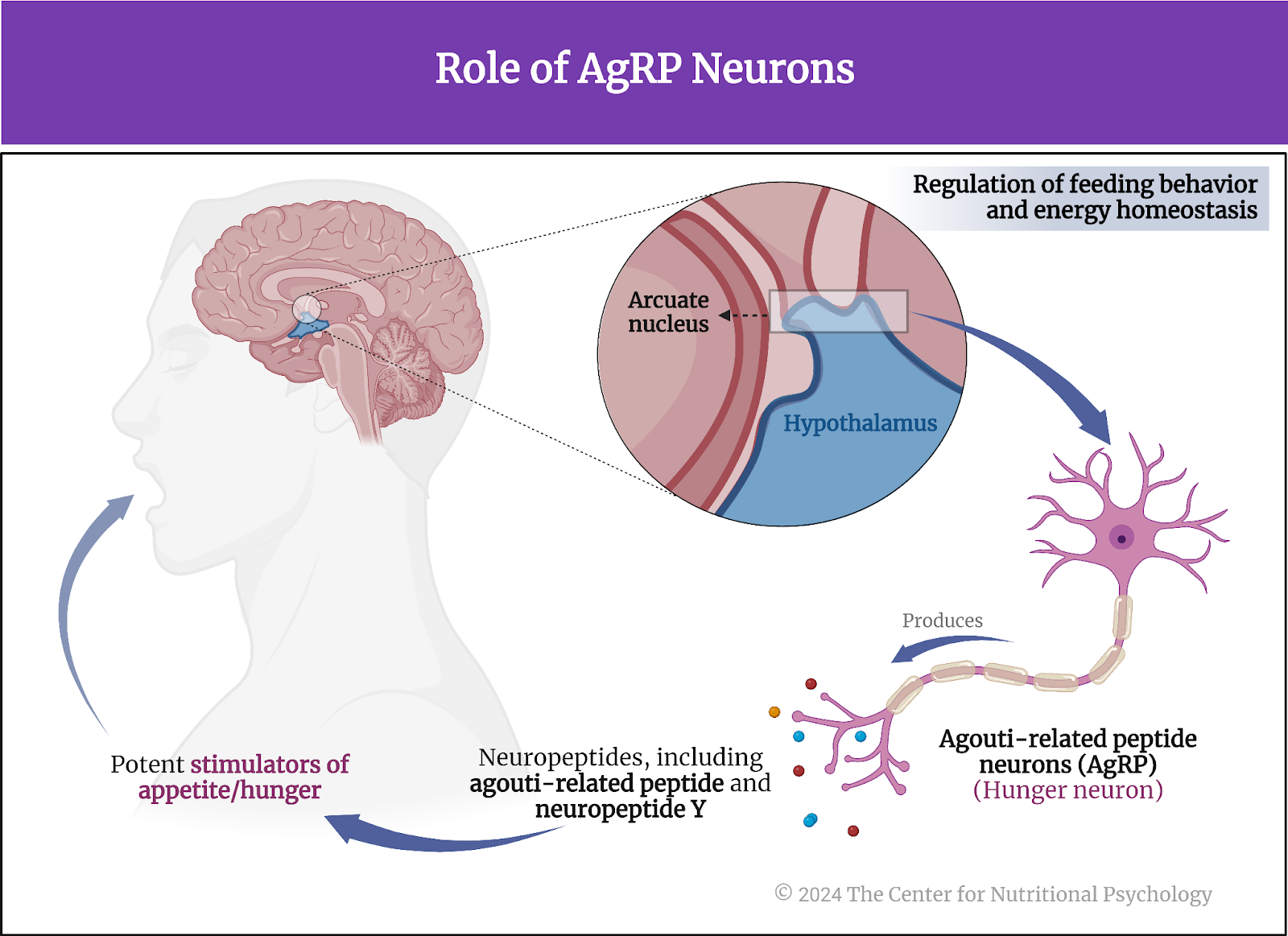
Figure 1. Role of AgRP neurons
The activity of AgRP neurons increases food intake and reduces energy expenditure, leading to weight gain. They are part of a complex neural network that responds to various signals related to energy status, such as hormones indicating hunger (ghrelin) or satiety (leptin and insulin). In conditions of energy deficit, such as fasting, AgRP neurons are stimulated, promoting hunger and encouraging food-seeking behavior (Atasoy et al., 2012; Chen et al., 2016).
“Hunger neurons” (AgRP) are a specific type of neuron located in the brain’s arcuate nucleus of the hypothalamus region and play a critical role in regulating feeding behavior and energy homeostasis
This system is crucial for survival, ensuring that energy intake is increased when energy stores are low. However, dysregulation of AgRP neurons and the pathways they influence can contribute to eating disorders and obesity (see Figure 2).
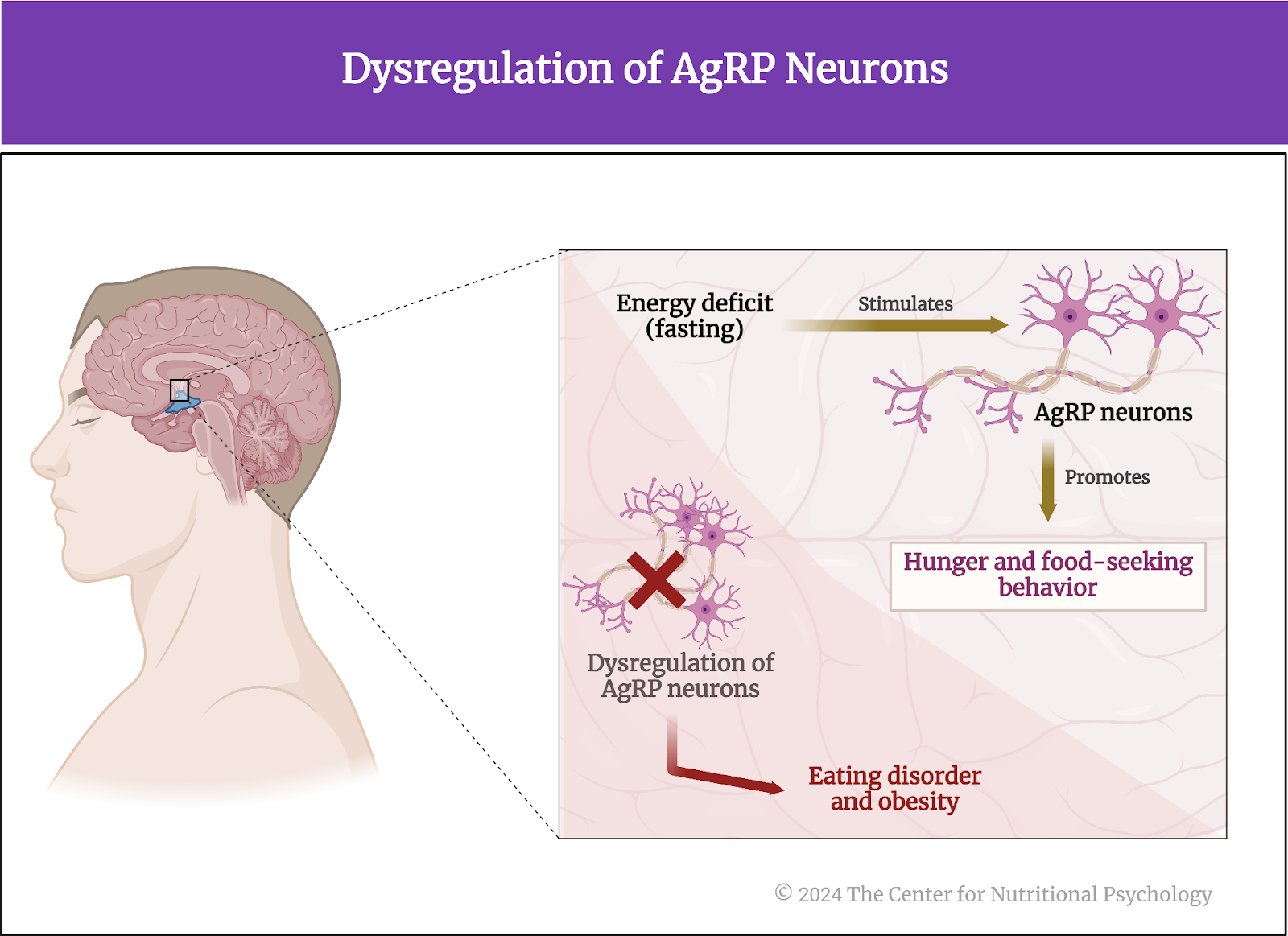
Figure 2. Dysregulation of AgRP neurons
The current study
Study author Michelle L. Klima and her colleagues wanted to map the neural pathways from the brain to the periphery that convey the anti-inflammatory effects of food deprivation. They note that it is likely that hunger circuits (AgRP neurons) in the central nervous system play a role in this because previous studies have shown that manipulations of gene expressions in these neurons can influence immune responses (Klima et al., 2023).
They conducted a series of experiments on mice. The mice used in this study were male and female, kept in group housing, and at least eight weeks old.
Hunger reduces inflammation in response to irritants
In the first experiment, study authors injected either formalin, complete Freund’s adjuvant (CFA), or a saline solution into the paws of these mice. Formalin and CFA are strong irritants that cause an inflammatory reaction and swelling of the paw. The saline solution injection was a control procedure. It is not expected to produce an inflammatory response, so the researchers use it as a reference for comparison with the reactions caused by the injections of the other two substances.
In the experiment, the study authors had two groups of mice – 8 mice that were kept without food for 24 hours before the experiment, i.e., hungry, and another eight that had free access to food. Some mice were injected with irritants and others with a saline solution within the two groups.
Results showed that the paws of mice injected with irritants developed strong inflammation, visibly increasing in size. However, this inflammatory reaction was attenuated by approximately 50% in hungry mice compared to mice fed normally. Female mice had stronger paw inflammation after the CFA injection than male mice, but the inflammation reduction effects of hunger were similar in both sexes.
Concentrations of inflammatory cytokine tumor necrosis factor-alpha, an indicator of inflammation, were much lower in hungry mice, confirming that hunger had a strong anti-inflammatory effect. Researchers also compared the anti-inflammatory effects of hunger with the effects of non-steroidal anti-inflammatory drugs ketoprofen and ketorolac. They found that hunger reduced paw inflammation 20% more than these two drugs (see Figure 3).
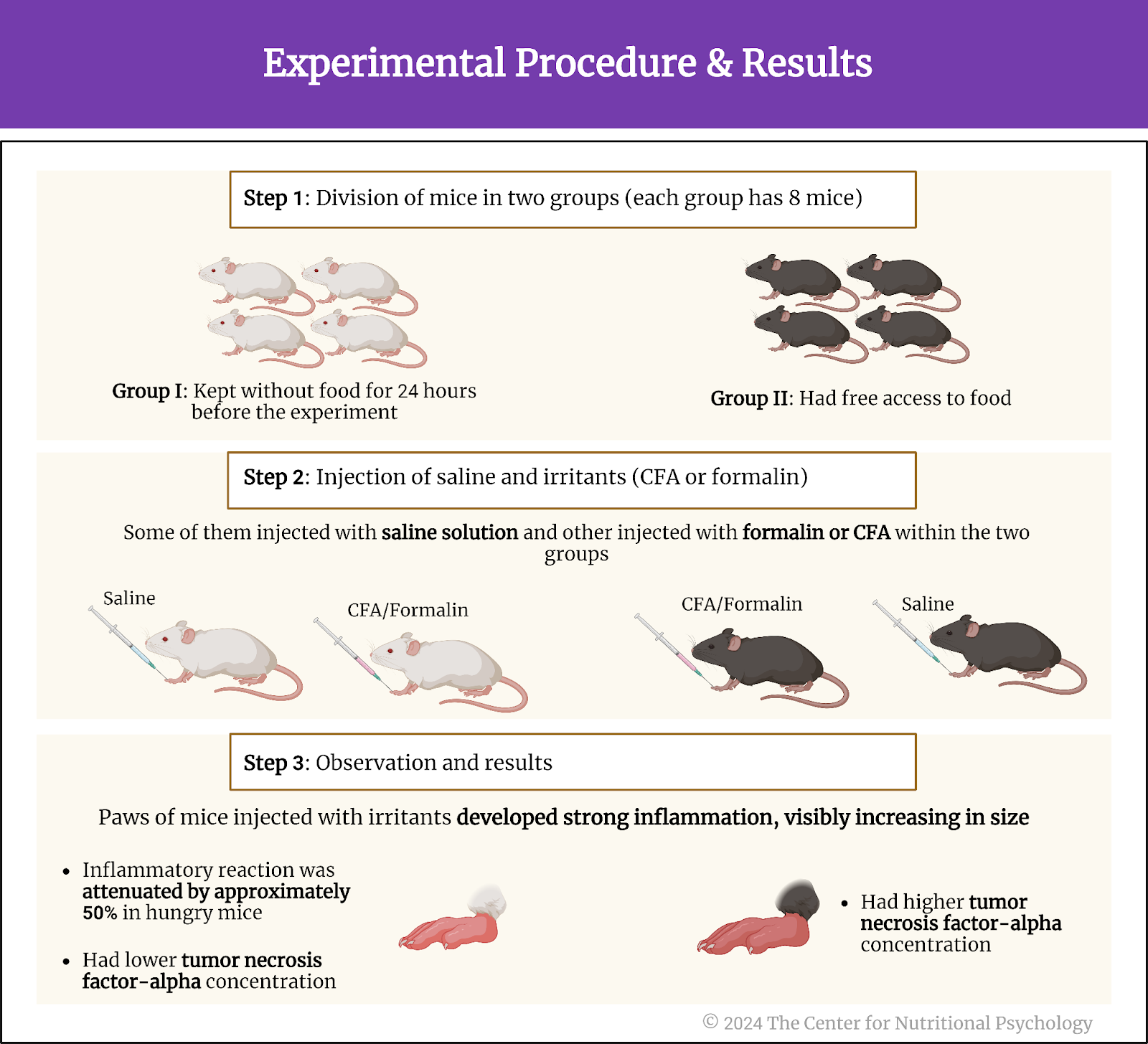
Figure 3. Experimental procedure and results
Anti-inflammatory effects of hunger go from the brain through the vagus nerve
In their next experiment, study authors disabled the vagus nerve of the mice either by cutting it below the diaphragm or by injecting chemicals to disable parts of this nerve.
The vagus nerve extends from the brainstem to the abdomen, innervating various organs. It is crucial in the parasympathetic nervous system, responsible for the body’s rest and digest functions. The vagus nerve helps regulate heart rate, digestion, and respiratory rate but is also involved in inflammatory responses. It also conveys sensory information from the internal organs to the brain.
Results showed that the anti-inflammatory effects of hunger were suppressed when the vagus nerve was disabled in this way. This happened both when the nerve was physically cut and when chemicals were used.
Further experimentation showed that the anti-inflammatory effect is suppressed only when neurons going from the brain to the periphery (efferent neurons) are destroyed or disabled. Destroying or disabling neurons that carry information from the periphery to the brain (afferent neurons) did not affect the anti-inflammatory effects of hunger. This told researchers that the anti-inflammatory effects of hunger go from the brain through the vagus nerve to other areas of the body (see Figure 4).
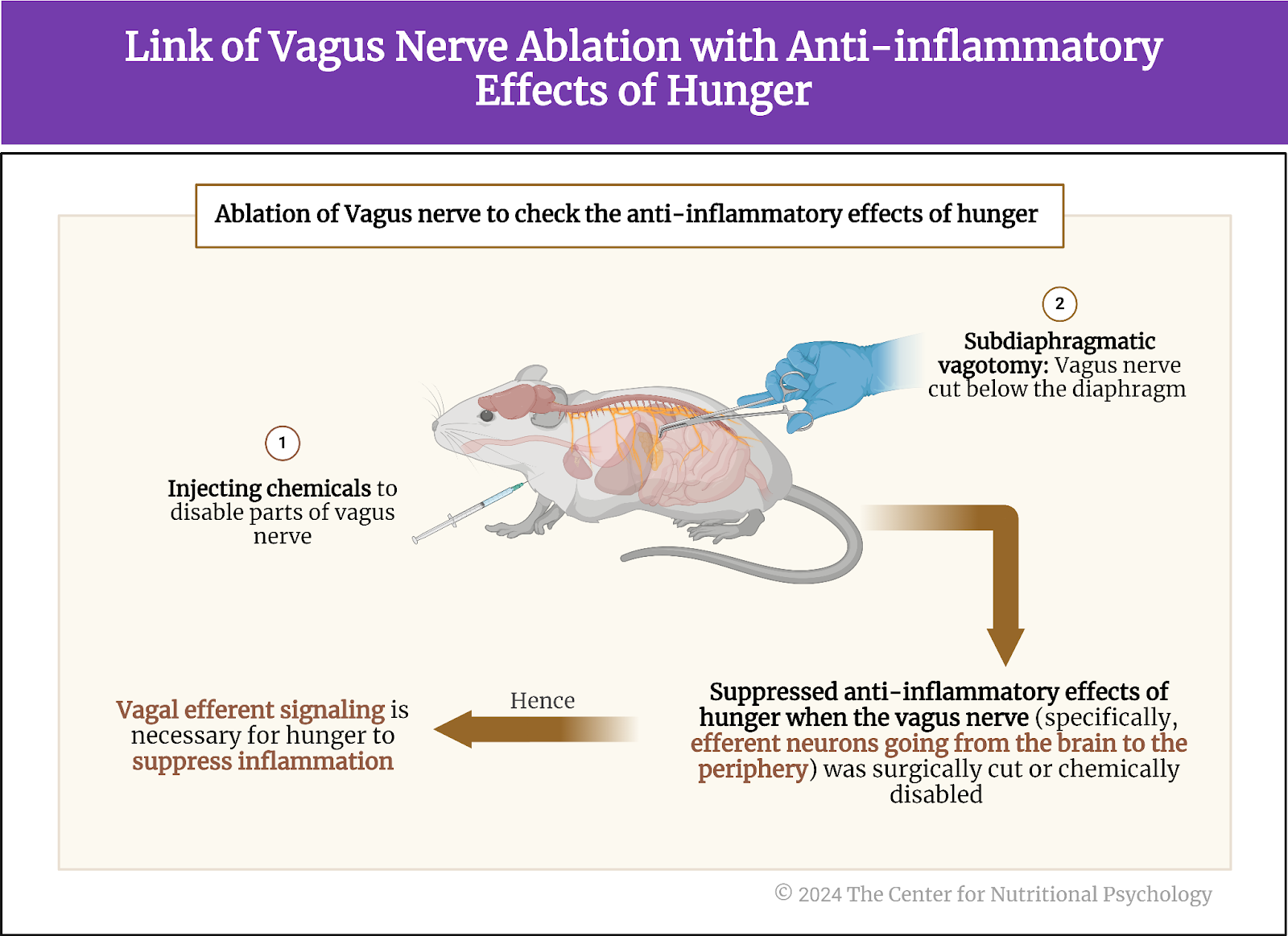
Figure 4. Link of vagus nerve ablation with anti-inflammatory effects of hunger
Activation of AgRP neurons reduces inflammation
Next, the study authors looked for neural circuits in the brain that trigger the anti-inflammatory response to hunger. Knowing that AgRP neurons are activated by hunger, they first looked at them. These researchers genetically modified the AgRP neurons in a group of mice to be activated by specific chemicals or light.
Results showed an anti-inflammatory response followed when they artificially activated these neurons without restricting food to mice. Paws of mice in which study authors artificially activated AgRP neurons showed a much less intense inflammatory reaction after an irritant (CFA) injection than mice from the control group that ate normally and whose AgRp neurons were not artificially activated. The anti-inflammatory reaction caused by the activation of AgRP neurons was rapid, taking less than 1 hour for the reduction in multiple indicators of inflammation in the paw to become visible (see Figure 5).
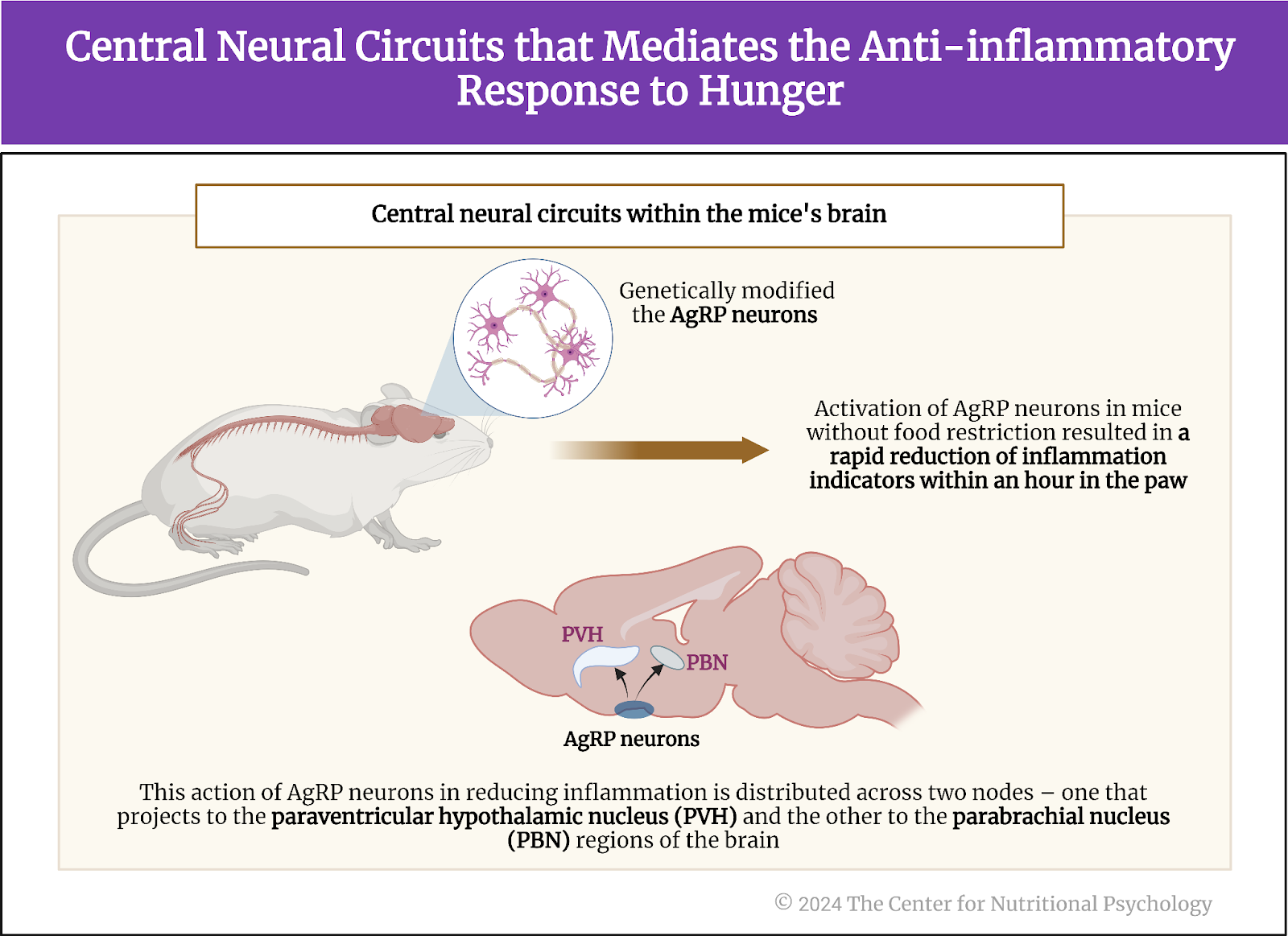
Figure 5. Central neural circuits that mediate the anti-inflammatory response to hunger
The study authors further sought to identify which AgRP neurons specifically activated the anti-inflammatory response. To do this, they activated specific groups of AgRP neurons one by one artificially in normally fed mice. They found that the anti-inflammatory action of AgRP neurons is distributed over two nodes – one projecting to the paraventricular hypothalamic nucleus and the other to the parabrachial nucleus regions of the brain. The magnitude of the anti-inflammatory effect of the first one was larger, mediating the bulk of the anti-inflammatory effects of these neurons.
Conclusions
Through a series of experiments on mice, this study showed that hunger produces an anti-inflammatory effect stronger than the effects of non-steroid anti-inflammatory medications such as ketoprofen and ketorolac. It showed that the bulk of the anti-inflammatory effect is mediated by hunger neurons, i.e., Agouti-related peptide (AgRP) neurons in the arcuate nucleus in the brain’s hypothalamus region. Even artificially triggering the activity of these neurons when a mouse is not hungry causes a rapid anti-inflammatory response.
These findings improve scientific understanding of the neural pathways of inflammation and can help healthcare researchers develop better ways to treat inflammation. Many diseases, from autoimmune diseases to skin disorders, are caused or aggravated by maladaptive immune responses of the body. Finding ways to better modulate or control the body’s immune response may be key to developing more effective treatments for them.
The paper “Anti-inflammatory effects of hunger are transmitted to the periphery via projection-specific AgRP circuits” was authored by Michelle L. Klima, Kayla A. Kruger, Nitsan Goldstein, Santiago Pulido, Aloysius Y.T. Low, Charles-Antoine Assenmacher, Amber L. Alhadeff, and J. Nicholas Betle.
References
Atasoy, D., Betley, J. N., Su, H. H., & Sternson, S. M. (2012). Deconstruction of a neural circuit for hunger. Nature, 488(7410), 172–177. https://doi.org/10.1038/nature11270
Chen, Y., Lin, Y.-C., Zimmerman, C. A., Essner, R. A., & Knight, Z. A. (2016). Hunger neurons drive feeding through a sustained, positive reinforcement signal. ELife, 5, e18640. https://doi.org/10.7554/eLife.18640.001
Dicker-Oren, S. D., Gelkopf, M., & Greene, T. (2022). The dynamic network associations of food craving, restrained eating, hunger and negative emotions. Appetite, 175(March), 106019. https://doi.org/10.1016/j.appet.2022.106019
Klima, M. L., Kruger, K. A., Goldstein, N., Pulido, S., Low, A. Y. T., Assenmacher, C. A., Alhadeff, A. L., & Betley, J. N. (2023). Anti-inflammatory effects of hunger are transmitted to the periphery via projection-specific AgRP circuits. Cell Reports, 42(11). https://doi.org/10.1016/j.celrep.2023.113338
Sidiropoulos, M. (2007). Anorexia Nervosa: The physiological consequences of starvation and the need for primary prevention efforts. CASE PRESENTATION. McGill Journal of Medicine, 10(1), 20–25.
Sternson, S. M., & Atasoy, D. (2014). Agouti-related protein neuron circuits that regulate appetite. Neuroendocrinology, 100, 95–102. https://doi.org/10.1159/000369072







Leave a comment